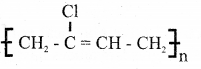Students can Download 2nd PUC Chemistry Previous Year Question Paper June 2019, Karnataka 2nd PUC Chemistry Model Question Papers with Answers helps you to revise the complete Karnataka State Board Syllabus and score more marks in your examinations.
Karnataka 2nd PUC Chemistry Previous Year Question Paper June 2019
Time: 3 Hrs 15 Min
Max. Marks: 70
Part-A
I. Answer all the questions each question carries one mark following: (10 × 1 = 10)
1.
Write the unit of molality of a solution?
Answer:
mol / kg-1 or mol / kg
2.
At a given temperature, oxygen gas is more soluble in water than Nitrogen gas. Which one of them has higher value of KH?
Answer:
N2 or Nitrogen
3.
State Faraday’s first law of electrolysis.
Answer:
The amount of substance discharged at any electrode during electrolysis is proportional to the quantity of electricity passed through the electrolyte.
4.
Give an example for pseudo first order reaction.
Answer:
Inversion of cane sugar OR
![]()
![]()
5.
Enthalpy of physical adsorption is quite low : Give reason.
Answer:
Because it involves weak van der Waals’ forces.
6.
Name the method used for concentration of sulphide ores.
Answer:
Froth Floatation Method or Process.
7.
Among Noble gases which one is most abundant in air?
Answer:
Argon
8.
Name the major organic product formed when 2 – bromopentane is heated with alcoholic potassium hydroxide solution.
Answer:
Pent – 2 – ene or 2 – Pentene
9.
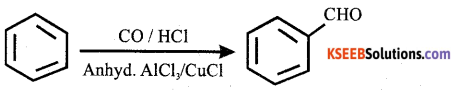
Name the above reaction.
Answer:
Gatterman – Koch reaction.
10.
Name the Hormone which regulates blood sugar level.
Answer:
Insulin or Glucagon.
Part-B
II. Answer any FIVE questions: (Each question carries two marks) (5 × 2 = 10)
11.
Which type of extrinsic semiconductor is formed when silicon is doped with phosphorus? Mention the major charge carrier in it.
Answer:
n – type, Electron (s).
12.
Draw a neat labeled diagram of Standard Hydrogen Electrode (SHE). Write its Half – Cell reaction.
Answer:
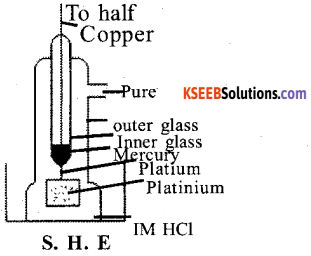
SHE is Symbolically represent as Pt, H2( 1 bar)/H+(1M)
![]()
13.
Name any two factors affecting the rate of a reaction.
Answer:
- Concentration of the reactants
- Temperature of the reactants
- Nature of the reacting substances
14.
(a) Zr and Hf have almost identical radii : Give reason.
Answer:
Due to Lanthanoid contraction.
(b) Name the gas liberated when Lanthanoids (Ln) react with acids.
Answer:
Hydrogen gas or H2 gas
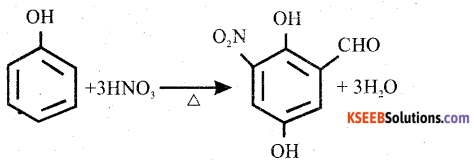
15.
How does phenol react with Cone. HNO3? Give equation.
Answer:
When phenol is heated with concentrated nitric acid, 2,4, 6 – trinitrophenol (picric acid) is formed.
16.
Explain Cannizzaro reaction with an example.
Answer:
Aldehydes with no α – hydrogen atom when treated with concentrated alkali (NaOH or KOH), undergo self – oxidation and reduction (disproporttionation) to give carboxylic acid salt and alcohol. This is called Cannizzaro’s reaction.
2HCHO + Conc.KOH → HCOOK + CH3OH
17.
What is the role of the following chemicals in food?
a) Sodiumbenzoate
Answer:
Food preservative
b) Saccharin
Answer:
Artificial sweetening agent.
![]()
18.
What is saponification? Write the equation to get sodium stearate by this method.
Answer:
When oil or fat is heated with aqueous NaOH, sodium salts of fatty acids (soaps) are formed.
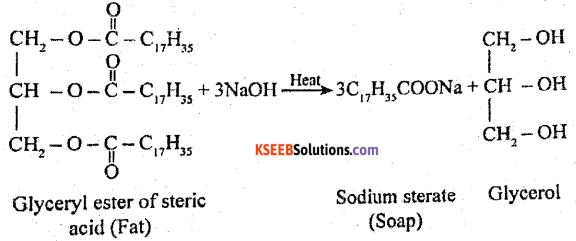
Part-C
III. Answer any FIVE of the following: (Each question carries three marks) (5 × 3 = 15)
19.
In the extraction of Aluminium by Hall – Herault process
a) Write the neat labeled diagram of electrolytic cell.
Answer:
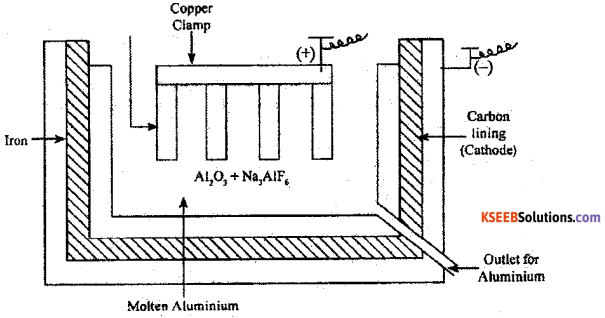
b) Give the equation of overall cell reaction.
Answer:
2Al2O3 + 3C → 4Al + 3CO2
c)
What is the role of cryolite?
Answer:
It lowers the melting point of the electrolyte (mix) and brings conductivity.
![]()
20.
In the manufacture of ammonia by Haber’s process. Write the flow chart and chemical equations with optimum conditions.
Answer:
Ammonia is manufactured by the direct combination of nitrogen and hydrogen in the ratio 1:3 by volume.
Reaction : N2 + 3H2(g) ⇌ N2NH3(g) ;
ΔH = -46 kJ/mol
According to Le-Chatelier’s principle, high yield of Ammonia is obtained under the following favourable conditions.
(a) High pressure of 200atm
(b) Temperature of ~ 700K
(c) Use of Iron oxide as Catalyst in presence of small amounts of K2O and Al2O3 as promoters.
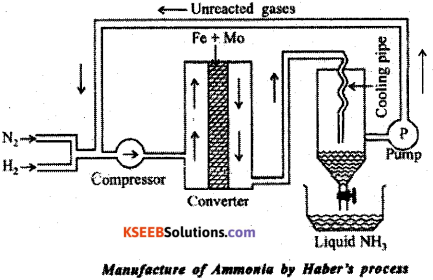
Procedure:
Step – 1 : Dry N2 and H2 in the ratio 1 : 3 compressed at 200 atm in compresser.
Step – 2 : Compressed gas passed through catalytical chamber, in presence of Fe, catalytical promoter Mo and 700 K forms ammonia.
Step – 3 : The ammonia formed is cooled in coolent below 239 K.
Step – 4 : The unreacted nitrogen and hydrogen are recirculated to compressor using pump. The above step repeated to get maximum yield.
21.
Complete the following equations :
a) 4AI + 3O2 →
b) PbS(S) + 4O3(g) →
c) C + 2H2SO4 →
(Conc.)
Answer:
a) 2Al2O3
b) PbSO4 + 4O2
c) 2H2O + CO2 + 2SO2
22.
a) Give any two reasons for anomalous behavior of Fluorine.
Answer:
- Ionization enthalpy, electro negativity and electrode potentials are all higher for fluorine than others.
- Hydrogen fluoride is a liquid due to strong hydrogen bonding. Other hydrogen halides are gases.
b) Write the structure of perchloric acid (HClO4).
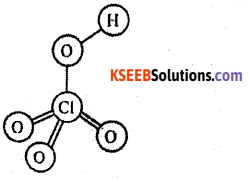
![]()
23.
a) Calculate the spin only magnetic moment of Ti3+ ion (Atomic number of Ti = 22).
Answer:
µ = \(\sqrt{n(n+2)}\) n = 1
µ = \(\sqrt{1(1+2)}\)
µ = \(\sqrt{3}\) =1.7 BM
b) Cu2+ salt solutions are coloured : Give reason.
Answer:
Due to the presence of unpaired electron or due to d – d transition of electrons.
24.
Write the balanced equations involved in the preparation of potassium dichromate (K2Cr2O7) from chromite ore.
Answer:
4FeCr2O4 + 8 Na2CO3 + 7O2 → 8Na2CrO4 +2Fe2O4 + 2Fe2O3 + 8CO2
OR
2Na2CrO4 + 2H+ → Na2Cr2O7 + 2Na+ + H2O
2Na2CrO4 + H2SO4 → Na2Cr2O7 + Na2SO4 + H2O
Na2Cr2O7 + 2KCl → K2Cr2O7 + 2NaCl.
25.
Explain the hybridization, geometry and magnetic property of [Co(NH3)6]3+ ion on the basis of Valance Bond Theory (VBT), (Atomic number of Co = 27].
Answer:
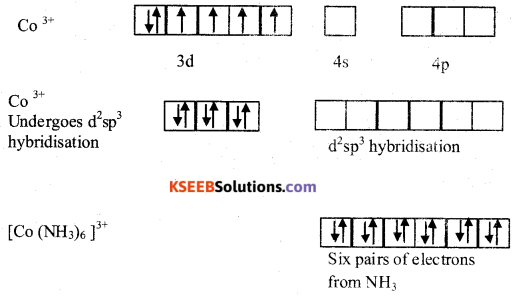
Geometry of [Co (NH3)6]3+ is Octahedral
Magnetic property: Diamagnetic
26.
a) For a given complex [Co(NH3)5NO2]Cl2. Write its IUPAC name and Linkage isomer.
Answer:
Pentaamminenitrito – N – Cobalt (III) chloride.
[CO(NH3)5 (ONO)]Cl2
b) Which set of d – orbitals of a metal atom / ion experience more repulsion in octahedral field created by the ligands?
Answer:
dx2 – y2 and dz2 orbitals.
![]()
Part-D
IV. Answer any three of the following: (Each question carries fire marks) (3 × 5= 15)
27.
(a) Calculate the packing efficiency in Face Centred Cubic (FCC) lattice.
Answer:
The number of atoms per unit cell in fCC structures is ‘4’
Each atom is considered as one sphere.
So the volume of 4 atoms
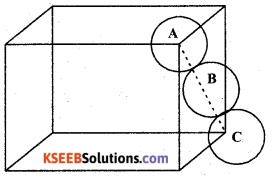
(4 sphere) = 4 × \(\frac { 3 }{ 4 }\) πr3 = \(\frac { 16 }{ 3 }\) πr3
Edge length a = 2\(\sqrt{2} r\)
The volume of the cubic unit cell = a3 = \((2 \sqrt{2} r)^{3}\)
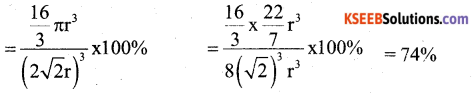
b) If a metal with atomic mass 209 crystallizes in a simple cubic lattice what is the edge length of its unit cell. (Given d = 91.5 kg m-3). 2
Answer:
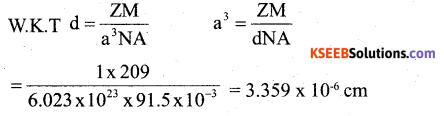
(c) Write any two differences between Frenkel and Schottkydefects.
Answer:
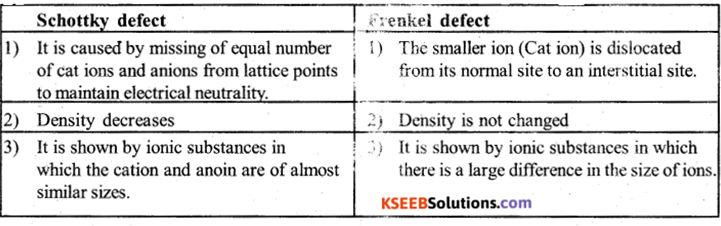
![]()
28.
(a) On dissolving 2.34g of non – electrolyte solute in 40g of benzene, the boiling point of solution was higher than benzene by 0.81 K. Kb value for benzene in 2.53 K kgmol-1. Calculate the molar mass of solute. [Molar mass of benzene is 78 gmol-1]
Answer:
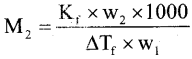
Kb = 2.53 K kg mol-1 w1 = 40g,
w2 = 2.34g, M2 = ?, ΔTb = 0.81 K
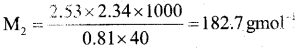
(b)
i) State Henry’s law.
Answer:
The partial pressure of the gas in vapor phase is proportional to the mole fraction of the gas in the solution.
ii) How solubility of a gas in liquid changes with increase in temperature?
Answer:
Decreases.
29.
(a) For a given data : E0Mg2+ /Mg = -2.37V E0Cu2+/Cu = +0.34V
Calculate the emf of the cell in which the following reaction takes place.
![]()
Answer:
E0cell = E0Cu2+/Cu – E0Mg2+ /Mg
= 0.34 – (-2.37) = 2.71V
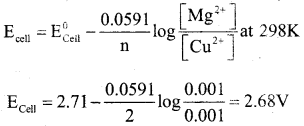
(b) State Kohlrausch law of independent migration of ions.
Answer:
The limiting molar conductivity of an electrolyte can be represented as the sum of the individual contributions of the anion and cation of the electrolyte.
c) Write the overall cell reaction in mercury cell.
Answer:
Zn(Hg) + HgO (s) → ZnO(s) + Hg(l)
![]()
30.
(a) Derive an integrated rate equation for the rate constant of a first-order reaction.
Answer:
consider a first order reaction
R → p
A/c to Rate law, Rate = k[R]1

where I is the integration constant
when t = 0, [R] = [R]0
In [R]0 = -k × o + I
I = In [R]0
where [R]0 is the initial con of the reactant ‘R’ substituting the value of I in equation (1)
we get In [R]0 = – kt + [R]0
kt = In [R]0 – In [R]
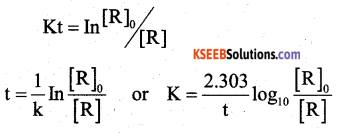
(b) The rate constant of a reaction is doubled when the temperature increased from 400K to 410 K. Calculate the activation energy (Ea) [R = 8.314 JK-1mol-1]
Answer:
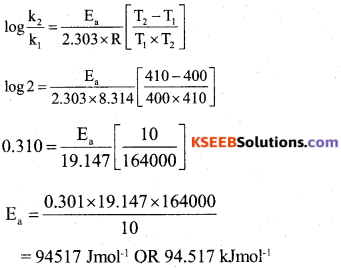
![]()
31.
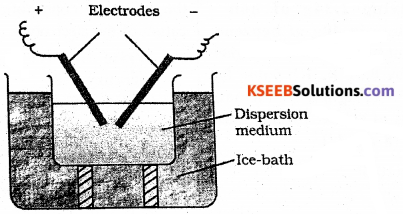
(a) How is Gold – Sol prepared by Bredig’s – arc method?
Answer:
This process involves dispersion as well as condensation. It is used to prepare sols of metals such as gold, silver, platinum etc.
- In this method, electric arc is struck between electrodes of the metal immersed in the dispersion medium contianing a little alkali.
- The intense heat produced vaporizes the metal which then condenses to form particles of colloidal size. The colloidal particles formed adsorb charges from the solution and get stabilized.
(b) What is Homogeneous catalysis? Give an example.
Answer:
A reaction in which all the reactants and the catalyst are in the same phase is called homogeneous catalysis.
![]()
(c) How does entropy change ( Δ S) for adsorption?
Answer:
Entropy decreases OR Δ S = -ve
![]()
V. Answer any four of the following. Each question carries five marks: (4 × 5 = 20)
32.
(a) Write the equations for the steps involved in SN1 mechanism for the conversion of tert – butyl bromide to tert – butyl alcohol.
Answer:
SN1 mechanism involves two steps
Step -1: Formation of carbocation:
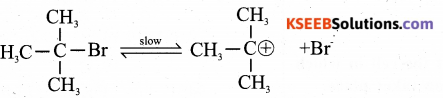
Step – 2: Attack of nucleophile on carbocation
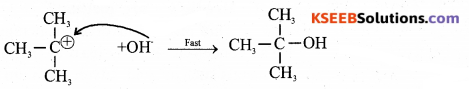
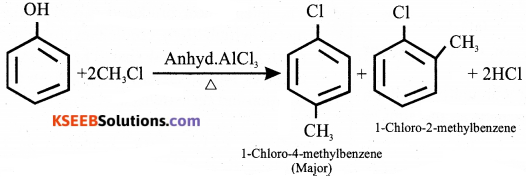
(b) Explain Friedel – Crafts alkylation for chlorobenzene. Give equation.
Answer:
An alkyl group can be added to a benzene molecule by an electrophile aromatic substitution reaction called the friedel – crafts alkylation reaction.
(c) What is recemic mixture?
Answer:
Equimolar mixture of dextrorotatory and laevorotatory isomers of the same compound is called racemic mixture.
33.
(a) Explain the mechanism of acid catalysed dehydration of ethanol to ethene.
Answer:
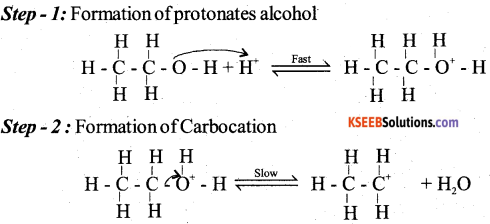
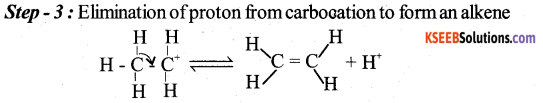
b) An organic compound with molecular formula C6H6O gives white precipitate with bromine water. Identify the functional group in the organic compound and write the chemical equation for the reaction.
Answer:
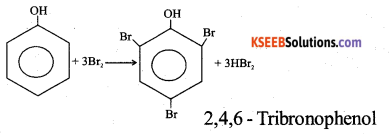
(c) Write general equation for preparation of ether by Williamson synthesis.
Answer:
R – X + R1ONa → R – O – R1 + NaX
(d) Among alcohols and phenols which one is more acidic?
Answer:
Phenol
![]()
34.
(a) Complee the following equations
![]()
Answer:
RCOOH
![]()
Answer:
CH4
(b) Explain Aldol condensation reaction for acetaldehyde. Write equation.
Answer:
A β – hydroxy aldehyde is known as aldol.
It is produced by the condesation reaction of two molecules of the same or one molecule each of two different aldehydes in the presence of a base.
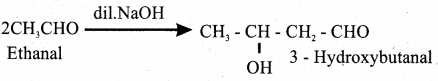
(c) What is Formalin solution?
Answer:
40% aqueous solution of formaldehyde.
35.
(a)How does Primary amine undergo carbyl amine reaction? Give chemical equation.
Answer:
Primary amines when heated with chloroform and alcoholic KoH give isoyanides having very unpleasant smell. The reaction is called as Carbylamine reaction.
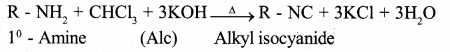
(b) How aniline is prepared by Hoffmann bromamide degradation reaction? Give equation.
Answer:
Benzamide is treated with bromine in aqueoues or ethanolic solution of sodium hydroxide to form aniline.
C6H5CONH2 + Br2+ 4 NaOH →
C6H5 NH2 + Na2CO3 + 2NaBr + 2H2O
OR
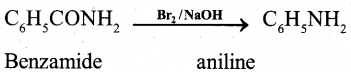
(c) Write the genera! formula of diazonium salt
Answer:
RN2+X– R= Aryl group OR ArN/X
![]()
36.
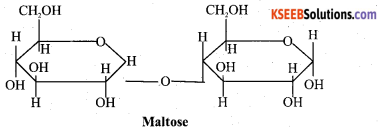
(a)Write the Haworth structure of maltose.
Answer:
(b) What are hormones? Give one biological function of insulin. 2
Answer:
Hormones are the biochemical messengers secreted by endocrine (ductless) glands.
Insulin regulates the blood sugar leveL
(c) What are nucleosides? 1
Answer:
The compounds containing pentose sugar and nitrogen base are called nucleosides.
(d) i) Name a naturally occuring oc-amino acid which is optically inactive.
Answer:
Glycine
ii) How many peptide bonds are present in a tripeptide?
Answer:
Two peptide bonds are formed.
(e) Deficiency of which Vitamin causes Night blindness?
Answer:
Vitamin – A
37.
(a)What is meant by polymerization? Name the monomer used in the preparation of Polyvinyl Chloride (PVC).
Answer:
The process of formation of polymers from respective monomers.
Vinyl chloride OR Chloroethene.
(b) Write partial structure of the following polymers,
i) Polypropene
Answer:
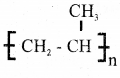
ii) Teflon
![]()
iii) Neoprene.