You can Download Chapter 5 Surface Chemistry Questions and Answers, Notes, 2nd PUC Chemistry Question Bank with Answers Karnataka State Board Solutions help you to revise complete Syllabus and score more marks in your examinations.
Karnataka 2nd PUC Chemistry Question Bank Chapter 4 Chemical Kinetics
2nd PUC Chemistry Surface Chemistry NCERT Textbook Questions and Answers
Question 1.
Distinguish between the meaning of the terms adsorption and absorption. Give one example of each.
Answer:
Adsorption is a surface phenomenon of accumulation of molecules of a substance at – the surface rather than in the bulk of a solid or liquid. The substance that gets adsorbed is called the ‘adsorbate’ and the substance on whose surface the adsorption takes place is called the “adsorbent”. Here, the concentration of the adsorbate on the surface of the adsorbent increases. In adsorption, the substances gets concentrated at the surface only.
It does not penetrate through the surface to the bulk of the solid or liquid for example, when we dip a chalk stick into an ink solution, only its surface becomes coloured. If we break the chalk stick, it will be found to be white from inside. On the other hand, the process of absorption is a bulk phenomenon. In absorption, the substance gets uniformly distributed throughout the bulk of the solid or liquid.
Question 2.
What is the difference between physisorption and chemisorption? ‘
Answer:
| Physisorption | Chemisorption |
| 1. In this type of adsorption, the adsorbate is attached to the surface of the absorbent with weak Van der Waal’s forces of attraction. | 1. In this type of adsorption, strong chemical bond is formed between the absorbate and the surface of the absorbent. |
| 2. No new compound is formed in the process | 2. New compounds are formed at the surface of the adsorbent. |
| 3. It is generally found to be reversible in nature. | 3. It is usually irreversible in nature. |
Question 3.
Give reason why a finely divided substance is more effective as an adsorbent.
Answer:
Adsorption is a surface phenomenon. Therefore, adsorption is directly proportional to the surface area. A finely divided substance has a large surface area. Both physisorption and chemisorption increase with an increase in the surface area. Hence, a finely divided substances behaves as a good absorbent.
![]()
Question 4.
What are the factors which influence the adsorption of a gas on a solid?
Answer:
There are various factors that affect the rate of adsorption of a gas on a solid surface.
- Nature of the gas: Easily liquefiable gases such as NH3, HCl etc. are adsorbed to a great extent in comparison to gases such as H2, O2 etc. This is because Van der Waal’s forces are stronger in easily liquefiable gases.
- Surface area of the solid: The greater the surface area of the adsorbent, the greater is the adsorption of a gas on the solid surface.
- Effect of pressure: Adsorption is a reversible process and is accompanied by a decrease in pressure. Therefore adsorption increases with an increase in pressure.
- Effect of temperature: Adsorption is an exothermic process. Thus in accordance with Leehatelie’s principle the magnitude of adsorption decreases with an increase in temperature.
Question 5.
What is an adsorption isotherm? Describe Freundlich adsorption isotherm.
Answer:
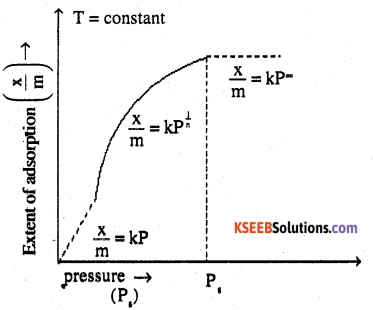
Freundlich adsorption isotherm:
Freundlich adsorption isotherm gives an empirical relationship between the quantity of gas adsorbed by the unit mass of solid absorbent and pressure at a specific temperature.
From the given plot it is clear that at pressure Ps, \(\frac { x }{ m }\) reaches the maximum value. Ps is called the saturation pressure. Three cases arise from the graph now.
Question 6.
What do you understand by activation of adsorbent? How is it achieved?
Answer:
By activating an absorbent, we tend to increase the adsorbing power of the adsorbent. Some ways to activate an adsorbent are:
1. By increasing the surface area of the adsorbent. This can be done by breaking it into smaller pieces or powdering it.
2. Some specific treatments can also lead to the activation of the adsorbent. For example, wood charcoal is activated by heating it between 650K and 1330K in vacuum pr air. It expels all the gases absorbed or adsorbed and thus, creates a space for adsorption of gases.
Question 7.
What role does adsorption play in heterogeneous catalysis?
Answer:
Heterogenous catalysis: A catalytic process in which the catalyst and the reactants are present in different phases is known as a heterogenous catalysis. This heterogenous catalytic action can be explained in terms of the adsorption theory. The mechanism of catalysis involves the following steps:
- Adsorption of reactant molecules on the catalyst surface.
- Occurrence of a chemical reaction through the formation of an intermediate.
- De-sorption of products from the catalyst surface.
- Diffusion of products away from the catalyst surface.
In this process, the reactants are usually present in the gaseous state and the catalyst is present in the solid state. Gaseous molecules are then adsorbed on the surface of the catalyst. As the concentration of reactants on the surface of the catalyst increases, the rate of reaction also increases. In such reactions, the products have less affinity for the catalyst and are quickly desorbed, thereby making the surface free for other reactants.
Case – I at low pressure:
The plot is straight and sloping, indicating that the pressure in directly proportional to
\(\frac { x }{ m }\) i.e., \(\frac { x }{ m }\) ∝ P
\(\frac { x }{ m }\)= kP (k = constant) m
Case – II At high pressure:
When pressure exceeds the saturated pressure,
becomes independent of P values.
\(\frac { x }{ m }\) ∝ P°
\(\frac { x }{ m }\) = kP°
Case – III At intermediate pressure:
X
At intermediate pressure, \(\frac { x }{ m }\) depends on P raised to the powers between O and ⊥ This relationship is known as the Freundlich adsorption isotherm.
\(\frac { x }{ m }\) ∝ P1/n
\(\frac { x }{ m }\) = KP1/n n > ⊥
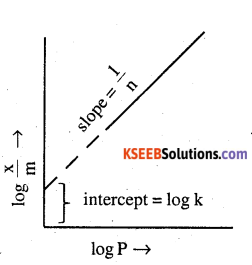
Now taking log:
log \(\frac { x }{ m }\) = log k + \(\frac { 1 }{ n }\) log P
on plotting the graph between log \(\frac { x }{ m }\) and logP, a straight line is obtained with the slope equal to \(\frac { 1 }{ n }\) and the intercept equal to log k.
![]()
Question 8.
Why is adsorption always exothermic ?
Answer:
Adsorption is always exothermic. This statement can be explained in two ways:
1. Adsorption leads to a decrease in the residual forces on the surface of the adsorbent. This causes a decrease in the surface energy of the adsorbent. Therefore, adsorption is always exothermic.
2. ∆H of adsorption is always negative. When a gas is adsorbed on a solid surface, its movement is restricted leading to a decrease in the entropy of the gas i.e. As is negative. Now for a process to be spontaneous, ∆G should be negative.
∴ ∆G = ∆n – T ∆s
since ∆s is negative, An has to be negative to make ∆G negative. Hence, adsorption is always expthermic.
Question 9.
How are the colloidal solutions classified on the basis of physical states of the dispersed phase and dispersion medium?
Answer:
One criterion for classifying colloids is the physical state of the dispersed phase and dispersion medium. Depending upon the type of the dispersed phase and dispersion medium (solid, liquid, or gas), there can be eight types of colloidal systems.
| Dispersed phase | Dispersion medium | Type of colloic | Example | |
| 1 | Solid | Solid | Solid soil | Gemstone |
| 2 | Solid | Liquid | Solid | paint |
| 3 | Solid | Gas | Aerosol | Smoke |
| 4 | Liquid | Solid | Gel | Cheese |
| 5 | Liquid | Liquid | Emulsion | Milk |
| 6 | Liquid | Gas | Aerosol | Fog |
| 7 | Gas | Solid | Solid, foam | Pumice, stone |
| 8 | Gas | Liquid | Foam | Froth |
Question 10.
Discuss the effect of pressure and temperature on the adsorption of gases on solids.
Answer:
Effect of pressure:
Adsorption is a reversible process and is accompanied by a decrease in pressure. Therefore adsorption increases with an increase in pressure.
Effect of temperature:
Adsorption is an exothermic process. Thus, in accordance with Le-chatelier’s principle, the magnitude of adsorption decreases with an increase in temperature.
Question 11.
What are lyophilic and lyophobic sols? Give one example of each type. Why are hydrophobic sols easily coagulated ?
Answer:
(i) Lyophilic Sols:
Colloidal sols that are formed by mixing substances such as gum, gelatin, starch etc. with a suitable liquid (dispersion medium) are called lyophilic sols. These sols are reversible in nature i.e. if two constituents of the sol are separated by any means (such as evaporation), then the sol can be prepared again by simply mixing the dispersion medium with the dispersion phase and shaking the mixture.
(ii) Lyophobic sols:
When substances such as metals and their sulphides etc. are mixed with the dispersion medium, they do not form colloidal sols. Their colloidal sols can be prepared only by special methods. Such sols are called lyophobic sols. These sols are irreversible in nature. For example: sols of metals.
Now, the stability of hydrophilic sols depends on two things the presence of a charge and the salvation of colloidal particles on the other hand, the stability of hydrophobic sols is only because of the presence of a charge. Therefore, the latter are very much less stable than the former. If the charge of hydrophobic sols is removed (by addition of electrolytes), then the particles present in them some closer and form aggregates leading to precipitation.
![]()
Question 12.
What is the difference between multimolecular and macromolecular colloids? Give one example of each. How are associated colloids different from these two types of colloids?
Answer:
(i) In multi-molecular colloids, the colloidal particles are an aggregate of atoms or small molecules with a diameter of less than ⊥ nm. The molecules in the aggregate are held together by Van der Waal’s forces of attraction. Example of such colloids include gold sol and sulphur sol.
(ii) In macro-molecular colloids, the colloidal particles are large molecules having colloidal dimensions. These particles have a high molecular mass. When these particles are dissolved in a liquid, sol is obtained. For example: Starch, nylon, cellulose, etc.
(iii) Certain substances tend to behave like normal electrolytes at lower concentration. However, at higher concentrations, these substances behave as colloidal solutions due to the formation of aggregated particles. Such colloids are called aggregated colloids.
Question 13.
What are enzymes ? Write in brief the mechanism of enzyme catalysis.
Answer:
Enzymes are basically protein molecules of high molecular masses. These form colloidal solutions when dissolved in water. These are complex, nitrogenous organic compounds produced by living plants and animals. Enzymes are also called ‘biochemical catalysts’.
E + S ⇌ [E -S] → E + P
Step -1 – Formation of enzyme – substrate complex
E+S ⇌ E – S
Step -2 – Dissociation of complex
E – S → [EP] → E+P(product)
Mechanism of enzyme catalysis:
On the surface of the enzymes, various cavities are present with characteristics shapes, these cavities process active groups such as NH2, -coon, etc. The reactant molecules having a complimentary shape fit into the cavities just like a key fits into a lock. This leads to the formation of an activated complex. This complex then decomposes to give the product.
Step 1: E + S → ES+
(Activated complex)
Step 2: ES+ → E + P
Question 14.
How are colloids classified on the basis of
(i) physical states of components
(ii) nature of dispersion medium and
(iii) interaction between dispersed phase and dispersion medium?
Answer:
Colloids can be classified on various bases:
(i) On the basis of the physical state of the components (by components we mean the dispersed phase and dispersion medium). Depending on whether the components are solids, liquids, or gases, we can have eight types of colloids.
(ii) On the basis of the dispersion medium, sols can be divided as
| Dispersion medium | Name of sol |
| Water | Aquasol or hydrosol |
| Alcohol | Alcosol |
| Benzene | Benzosol |
| Gases | Aerosol |
(iii) On the basis of the nature of the interaction between the dispersed phase and dispersion medium, the colloids can be classified as lyophilic (solvent attracting) and.lyophobic (solvent repelling).
Question 15.
Explain what is observed
(i) when a beam of light is passed through a colloidal sol.
(ii) an electrolyte, NaCl is added to hydrated ferric oxide sol.
(iii) electric current is passed through a colloidal sol?
Answer:
(i) When a beam of light is passed through a colloidal solution, then scattering of light is observed. This is known as the Tyndall effect. This scattering of light illuminates the path of the beam in the colloidal solution.
(ii) When NaCl is added to ferric oxide sol, it dissociates to give Na+ and Cl– ions. Particles of ferric oxide sol are
positively charged. Thus, they get coagulated in the presence of negatively charged Cl– ions.
(iii) The colloidal particles are charged and carry either a positive or negative charge. The dispersion medium carries an equal and opposite charge. This makes the whole system neutral. Under the influence of an electric current, the colloidal particles move towards the oppositely charged electrode. When they come in contact with the electrode they lose their charge and coagulate,
Question 16.
What are emulsions? What are their different types? Give example of each type.
Answer:
The colloidal solution in which the dispersed phase and dispersion medium are liquids is called an emulsion.
There are two types of emulsions:
(a) Oil in water type:
Here, oil is the dispersed phase while water is the dispersion medium. For example milk, vanishing cream, etc.
(b) Water in oil type:
Here, water is the dispersed phase while oil is the dispersion medium. For example cold cream, butter, etc.
![]()
Question 17.
What is demulsification? Name two demulsifiers.
Answer:
The process of decomposition of an emulsion into its constituent liquids is called demulsification. Examples of demulsifiers are surfactants, ethylene oxide, etc.
Question 18.
Action of soap is due to emulsification and micelle formation. Comment.
Answer:
The cleansing action of soap is due to emulsification and micelle formation, Soaps are basically sodium and potassium salts of long chain fatty acids, R-COO–Na+. The end of the molecule to which the sodium is attached is polar in nature, while the aky 1-end is non-polar.
Thus, a soap molecule contains a hydrophilic (polar) and a hydrophobic (non polar) part.
When soap is added to water containing dirt, the soap molecules surround the dirt particles in such a manner that their hydrophobic parts get attached to the dirt molecule and the hydrophilic parts point away from the dirt molecules. This is known as micelle formation. Thus we can say that the polar group dissolves in water while the non-polar group dissolves in the dirt particle.
Now, as these micelles are negatively charged, they do not coagulate and a stable emulsion is formed.
Question 19.
Give four examples of heterogeneous catalysis.
Answer:
(i) Oxidation of sulphur dioxide to form sulphur trioxide. In this reaction, Pt acts as a catalyst.
![]()
(ii) formation of ammonia by the combination of dinitrogen and dihydrogen in the presence of finely divided iron.
![]()
This is called the Haber’s process.
(iii) Oswald’s process: Oxidation of ammonia to nitric oxide in the presence of platinum.
![]()
(iv) Hydrogenation of vegetable oil in the presence of Ni Vegetable oil (1) + H2(g)
Question 20.
What do you mean by activity and selectivity of catalysts?
Answer:
(a) Activity of a catalyst:
The activity of a catalyst is its ability to increase the rate of a particular reaction. Chemisorption is the main factor in deciding the activity of a catalyst. The adsorption of reactants on the catalyst surface should be neither too strong nor too weak. It should just be strong enough to make the catalyst active.
(b) Selectivity of the catalyst:
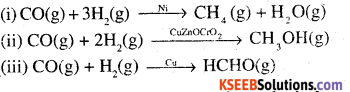
The ability of the catalyst to direct a reaction to yield a particular product is refered to as the selectivity of the catalyst. For example, by using different catalysts, we can get different products for the reaction between H2 and CO.
Question 21.
Describe some features of catalysis by zeolites.
Answer:
Zeolites are aluminosilicates that are micro-pores in nature. Zeolites have a honeycomb-like structure, which makes them shape-selective catalysts. They have an extended 3D network of silicates in which some silicon atoms are replaced by aluminium atoms, giving them an Al-O-Si framework. The reactions taking place in zeolites are very sensitive to the pores and cavity size of the zeolites. Zeolites are commonly used in the petrochemical industry.
![]()
Question 22.
What is shape selective catalysis?
Answer:
A catalytic reaction which depends upon the pore structure of the catalyst and on the size of the reactant and the product molecules is called shape-selective catalysis. For examples, catalysis by zeolites is a shape- selective catalysis. The pore size present in the zeolites ranges from 260-740 pm. Thus, molecules having a pore size more than this cannot enter the zeolite and undergo the reaction.
Question 23.
Explain the following terms:
(i) Electrophoresis
(ii) Coagulation
(iii) Dialysis
(iv) Tyndall effect.
Answer:
(i) Electrophoresis:
The movement of colloidal particles under the influence of an electric field is known as electrophoresis. Positively charged particles move to the cathode, while negatively charged particles move towards the anode. As the particles reach oppositely charged electrodes, they become neutral and get coagulated.
(ii) Coagulation:
The process of setting down of colloidal particles i.e., conversion of a colloid into a precipitate is called coagulation.
(iii) Dialysis: The process of removing a dissolved substances from a colloidal solution by the means of diffusion through a membrane is known as dialysis. This process is based on the principle that ions and small molecules can pass through animal membranes unlike colloidal particles.
(iv) Tyndall effect:
When a beam of light is allowed to pass through a colloidal solution, it becomes visible like a column of light. This is known as the Tyndall effect. This phenomenon takes place as particles of colloidal dimension scatter light in all directions. .
Question 24.
Give four uses of emulsions.
Answer:
Four uses of emulsions:
- cleansing action of soaps is based on the formation of emulsions
- digestion of fats in intestines takes place by the process of emulsification.
- Antiseptics and disinfectants when added to water form emulsions.
- The process of emulsification if sed to make medicines.
Question 25.
What are micelles? Give an example of a micellers system.
Answer:
Micelle formation is done by substances such as soaps and detergents when dissolved in water. The molecules of such substances contains a hydrophobic and a hydrophilic part when present in water, these substances arrange themselves in spherical structures in such a manner that their hydrophobic parts are present towards the centre, while the hydrophilic parts are pointing towards the outside. This is known as micelles formation.
Question 26.
Explain the terms with suitable examples:
(i) Alcosol
(ii) Aerosol
(iii) Hydrosol.
Answer:
(i) Alcosol:
A colloidal solution having alcohol as the dispersion medium and a solid substance as the dispersed phase is called an alcosol.
(ii) Aerosol:
A colloidal solution having a gas as the dispersion medium and a solid as the dispersed phase is called an aerosol.
For e.g.: Fog.
(iii) Hydrosol:
A colloidal solution having water as the dispersion medium and a solid as the dispersed phase is called a hydrosol.
For example: starch sol or gold sol.
![]()
Question 27.
Comment on the statement that “colloid is not a substance but a state of substance”.
Answer:
Common salt (atypical crystalloid in an aqueous medium) behaves as a colloid in a benzene medium. Hence, we can say that a colloidal substance does not represent a separate class of substances. When the size of the solute particle lies between lnm and 1000 nm, it behaves as a colloid.
Hence, we can say that colloid is not a substance but a state of the substance which is dependent on the size of the particle.
A colloidal state is intermediate between a true solution and a suspension.
Question 28.
Write any two characteristics of chemisorption.
Answer:
- Chemisorption is highly specific in nature. It occurs only if there is a possibility of ‘ chemical bonding between the adsorbent and the adsorbate.
- like physisorption, chemisorption also increases with an increase in the surface area of the adsorbent.
Question 29.
Why does physisorption decrease with the increase of temperature?
Answer:
Physisorption is exothermic in nature. Therefore, in accordance with Le-Chateliere’s principle, it decreases with an increase in temperature. This means that physisorption occurs more readily at a lower temperature.
Question 30.
Why are powdered substances more effective adsorbents than their crystalline forms?
Answer:
Powdered substances are more effective adsorbents than their crystalline forms because when a substance is powdered, its surface area increase and physisorption is directly proportional to the surface area of the adsorbent.
Question 31.
Why is it necessary to remove CO when ammonia is obtained by Haber’s process?
Answer:
It is important to remove CO in the synthesis of ammonia as CO adversely affects the activity of the iron catalyst, used in Haber’s process.
Question 32.
Why is the ester hydrolysis slow in the beginning and becomes faster after sometime?
Answer:
Ester hydrolysis can be represented as: Ester + water → Acid + Alcohol The acid produced in the reaction acts as a catalyst and makes the reaction faster, substances that act as catalysts in the same reaction in which they are obtained as products are known as anticatalysts.
Question 33.
What is the role of desorption in the process of catalysis?
Answer:
The role of desorption in the process of catalysis is to make the surface of the solid catalyst free for the fresh adsorption of the reactants on the surface.
Question 34.
What modification can you suggest in the Hardy-schulze law?
Answer:
Hardy-Schulze law states that the greater the valence of the flocculating ion added, the greater is its power to cause precipitation. This law takes into consideration only the charge carried by an ion, not its size. The smaller the size of an ion, the more will be its polarising power. Thus, Hardy-Schulze law can be modified in terms of the polarising power of the flocculating ion. Thus, the modified Hardy- Schulze law can be stated as the greater the polarising power of the flocculating ion added, the greater is its power to cause precipitation.
Question 35.
Why is it essential to wash the precipitate with water before estimating it quantitatively?
Answer:
When a substance gets precipitated, some ions that combine to form the precipitate get adsorbed on the surface of the precipitate. Therefore, it becomes important to wash the precipitate before estimating it quantitatively in order to remove these adsorbed ions or other such impurities.
2nd PUC Chemistry Surface Chemistry Additional Questions and Answers
Question 1.
What causes Brownian movement in a colloidal solution? (Delhi 2008)
Answer:
The molecules of dispersion medium due to their kinetic motion strike against the colloidal particles (dispersed phase) from all sides with different forces causing them to move.
Question 2.
What are Lyophilic and Lyophobic sols? Give example of each type. Which one of these two types of sols is easily coagulated and why? (Delhi 2008)
Answer:
(i) Lyophilic Sols:
Colloidal sols that are formed by mixing substances such as gum, gelatin, starch etc. with a suitable liquid (dispersion medium) are called lyophilic sols. These sols are reversible in nature i.e. if two constituents of the sol are separated by any means (such as evaporation), then the sol can be prepared again by simply mixing the dispersion medium with the dispersion phase and shaking the mixture.
(ii) Lyophobic sols:
When substances such as metals and their sulphides etc. are mixed with the dispersion medium, they do not form colloidal sols. Their colloidal sols can be prepared only by special methods. Such sols are called lyophobic sols. These sols are irreversible in nature. For example: sols of metals.
Now, the stability of hydrophilic sols depends on two things the presence of a charge and the salvation of colloidal particles on the other hand, the stability of hydrophobic sols is only because of the presence of a charge. Therefore, the latter are very much less stable than the former. If the charge of hydrophobic sols is removed (by addition of electrolytes), then the particles present in them some closer and form aggregates leading to precipitation.
![]()
Question 3.
Which has a higher enthalpy of adsorption, physisorption or chemisorption? (Delhi 2008)
Answer:
Enthalpy of chemisorption is high (80-240 KJ mol-1) as it involves chemical bond formation. .
Question 4.
Explain what is observed when
(i) an electrolyte, KCl, is added to hydrated ferric oxide solution .
(ii) an electric current is passed through a colloidal solution
(iii) a beam of strong light is passed through a colloidal solution (Delhi 2008, AI2008)
Answer:
(i) When an electrolyte like KCl is added of Fe (OH)3sol, the positively charged colloidal particles of Fe(OH)3 get coagulated by the oppositely charged Cl ions provided by KCl
(ii) On passing the electric current, colloidal particles move towards the oppositely charged electrode where they lose their charge and get coagulated.
(iii) When a beam of strong light is passed through a colloidal solution scattering of light by colloidal particles takes place and the path of light becomes visible. This phenomenon is called Tyndall effect.
![]()
Question 5.
(a) What are zeolites? Give any one of its use.
(b) A U-tube is filled with ferric hydroxide solution. Two platinum electrodes are introduced into the two limbs of the U-tube and an electric current is passed through the electrodes. What do you observe? Name the phenomenon.
Answer:
(a) Zeolites are aluminosilicates having three dimensional structure. Zeolites are highly porous have cavities of different sizes. The catalytic behaviour of zeolite catalysts depends upon the size of the cavities in them, which usually varies from 260-740 pm. The reaction molecules of a particular shape and size can only enter and get absorbed. Thus Zeolites are called shape selective catalysts.
(b) Colloidal particles move towards one or other electrode. Phenomenon is called electrophoresis.
Question 6.
Classify the following into homogenous and
heterogenous catalysis
(i) Catalytic decomposition of ozone by chlorine
(ii) Hydrolysis of an organic ester
(iii) Haber’s process.
Answer:
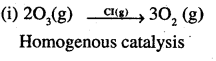
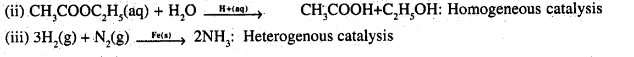
Question 7.
It is found that when Litmus is shaken with animal charcoal adsorption takes place
(i) Find out the adsorbent and adsorbate in this process.
(ii) explain the terms adsorbent and adsorbate.
(iii) Name the protect of removing adsorbate from adsorbent.
(iv) Explain the term “sorption’.
Answer:
(i) Litmus is the adsorbate and charcoal is the adsorbent.
(ii) The material providing the surface upon which absorption occurs, is known as the adsorbent and the substance adsorbed is called adsorbate.
(iii) The process of removal of adsorbed substance from the surface of the adsorbent is called desorption.
(iv) The phenomenon in which absorption and adsorption occur together is called sorption.
Question 8.
Write any two characteristics of chemisorption.
Answer:
- Chemisorption is highly specific
- Chemisorption involves compound formation and hence is irreversible in nature.
Question 9.
Why is ester hydrolysis slow in the beginning and becomes faster after sometime?
Answer:
When an ester is treated with mineral acid, gives an acid and alcohol. The organic acid undergoes dissociation to give hydrogen ions. These hydrogen ions act as catalyst and hence and the hydrolysis becomes faster.
Question 10.
What is the role of desorption in the process of catalysis?
Answer:
The product formed during catalysis gets detached from the surface at the catalyst by the process of desorption and makes available the catalyst surface for move reaction to occur.
![]()