You can Download Chapter 6 General Principles and Processes of Isolation of Elements Questions and Answers, Notes, 2nd PUC Chemistry Question Bank with Answers Karnataka State Board Solutions help you to revise complete Syllabus and score more marks in your examinations.
Karnataka 2nd PUC Chemistry Question Bank Chapter 6 General Principles and Processes of Isolation of Elements
2nd PUC Chemistry General Principles and Processes of Isolation of Elements NCERT Textbook Questions and Answers
Question 1.
Copper can be extracted by hydrometallurgy but not zinc. Explain.
Answer:
The E° of zinc (Zn2+ / Zn = -0.76v) or iron (Fe2+ / Ee = -0.44v) is lower than that of copper (Cu2+ / Cu = + 0.34v) and hence both Zn and Fe, can displace Cu from solutions of Cu2+ ions. Although zinc is a stronger reducing agent than iron, yet iron scrap is chiefty used in hydrometallurgy of copper because it is much cheaper than zinc.
Fe (s) + Cu2+ (aq) → Fe2+ (aq) + Cu (s)
In contrast, to displace zinc from solution of Zn2+ ions, we need more reactive metal than it, i.e A1 (E° Al3+/ Al = -1.66v), Mg (e ° Mg2+ / Mg = -2.37v), Ca (e ° Ca2+ / Ca = -2.87) K . (e ° k+ / k = -2.93 v) etc.
But all these metals react with water [2H2 + 2e– → H2(g) + 20n- (ag), = -0.83v] forming their corresponding ions with the evolution of H2 gas.
Thus, Al, Mg etc cannot be used to displace zinc from solution of Zn2+ ions. Thus copper can be extracted by hydrometallurgy but not zinc.
Question 2.
What is the role of depressant in froth floatation process?
Answer:
In froth floatation-process, the role of the depressant is to prevent one type of sulphide ore particles from forming the froth with air bubbles. For eg. NaCN is used as a depressant to separate lead sulphide (PbS) ore from zinc sulphide (ZnS) ore. The reason being that NaCN forms a zinc complex, Na2[Zn (CN)4] on the surface of ZnS thereby preventing from the formation of the froth. Under these conditions, only PbS forms froth and hence can be separated from ZnS ore.
4 NaCN + ZnS → Na2 [Zn (CN)4] + Na2S
Sod. tetracy emozincate (II)
![]()
Question 3.
Why is the extraction of copper from pyrites more difficult than that from its oxide ore through reduction?
Answer:
The standard free energy (∆f G°) of formation of Cu2S is greater than those of CS2 and H2S (CS2 is infact, an endothermic compound) Therefore neither carbon nor hydrogen can reduce Cu2S to Cu metal.
Cu2S + H2 > 2Cu + H2S
2Cu2S + C > 4Cu + CS2
In contrast, ∆fG° of Cu20 is much lower than that of CO and hence carbon can easily reduce Cu2O to Cu.
Cu2O (S) + C (S) → 2 Cu (S) + CO (g)
It is because of this reason that the extraction of copper from pyrite is difficult than from its oxide ore through reduction.
Question 4.
Explain: (i) Zone refining (ii) Column chromatography.
Answer:
(i) This method is based on the principle that the impurities are more soluble in the molten state of metal (the melt) than in the solid state. In the process of zone refining, a circular mobile heater is fixed at one end of a rod of impure metal. As the heater moves the molten zone of the rod also moves with it. As a result pure metal crystallizes out of the melt and the impurities pass onto the adjacent molten zone. This process is repeated several times, which leads to segregation of impurities at one end of the rod. Then the end with impurities is cut off. Silicon, boron, gallium, indium etc can be purified by this process.
(ii) Column chromatography is a technique used to separated components of mixture where components are in minute quantities. In chromatography there are two phases : mobile phase and stationary phase. The stationary phase is immobile and immiscible. Al2O3 column is usually used as the stationary phase in column chromatography. The mobile phase may be a gas, liquid or super critical fluid in which the sample extracts dissolves. Then the mobile phase is forced to wave through stationary phase. The component that is more strongly absorbed on the column takes a longer time to travel than the component weakly , absorbed.
Question 5.
Out of C and CO, which is a better reducing agent at 673 K ?
Answer:
At 673K, the value of ∆G (CO, CO2) is less than that of ∆G (C, CO) therefore, CO can be reduced more easily to CO2 than C to , CO. Hence CO is a better reducing agent than ‘ C at 673K.
Question 6.
Name the common elements present in the anode mud in electrolytic refining of copper. Why are they so present ?
Answer:
The common elements present in the anode mud are antimony, selenium, tellurium, silver, gold and platinum. These elements, being less reactive, are not affected by CuSO4 – H2SO4 solution and hence settle down under anode as anode mud.
![]()
Question 7.
Write down the reactions taking place in different zones in the blast furnace during the extraction of iron.
Answer:
At 500 – 800 K (lower temperature range ‘ in the blast furnace) – .
3 Fe2O3 + CO → 2 Fe3O4 + CO2 (6.28)
Fe3O4 + 4 CO → 3Fe + 4 CO2 (6.29)
Fe2O3 + CO → 2 FeO + CO2 (6.30)
At 99 – 1500 K (higher temperature range in the blast furnace):
C + CO2 → 2 CO (6.31)
FeO + CO → Fe + CO2 (6.32)
Question 8.
Write chemical reactions taking place in the extraction of zinc from zinc blende.
Answer:
Various steps involve are :
(a) Concentration – The ore is crushed and then concentrated: froth floation process.
(b) Roasting – The concentrated ore is then roasted in presence of excess of air at about 1200k when zinc oxide (ZnO) is formed.
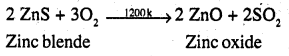
(c) Reduction – Zinc oxide obtained above is mixed with powdered coke and heated to 1673K in a fire clay retort when it is reduced to Zinc metal.
![]()
At 1673 k, zinc metal being volatile (b.p. 1180 K), distills over and is condensed.
(d) Electrolytic refining – Impure zinc is made the anode while cathode consists of a sheet of pure zinc. The electrolyte consists of ZnSO4 solution acidified with dil H2SO4. On passing current pure Zn gets deposited on the cathode.
Question 9.
State the role of silica in the metallurgy of copper.
Answer:
During roasting, copper pyrites are converted into mixture of FeO and Cu2O,
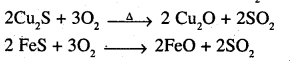
To remove FeO (basic) an acidic flux silica is added during smelting. FeO then combines with SiO2 to form ferrous silicate (FeSi03) slag which floats over molten matter.
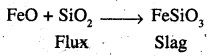
Thus, the role of silica in the metallurgy of copper is to remove iron oxide as slag.
Question 10.
What is meant by the term “chromatography”?
Answer:
The term chromatography was originally derived from the Greek word chroma meaning colour and graphy meaning writing because the method was first used for the separation of coloured substances (plant pigments) into individual components. Now the term chromatography has lost its original meaning and the method is widely used for separation, purification and characterization of the components of a mixture whether coloured or colourless.
Question 11.
What criterion is followed for the selection of the stationary phase in chromatography?
Answer:
The stationary phase is selected in such a way that the impurities are more strongly absorbed or are more soluble in the stationary phase than element to be purified. Under these conditions, when the column is extracted, the impurities will be retained by the stationary phase while the pure components is easily eluted.
![]()
Question 12.
Describe a method for refining nickel.
Answer:
When impure nickel is heated in a current of CO at 330 – 350K, it forms volatile nickel tetracarbonyl leaving behind the impurities. The nickel tetracarbonyl thus obtained is then heated to a higher temperature (450 – 470K), when it undergoes thermal decomposition to give pure nickel.
Question 13.
How can you separate alumina from silica in a bauxite ore associated with silica? Give equations, if any.
Answer:
Pure alumina may be separated from bauxite by Baeyer’s process as discussed below. The I bauxite ore associated with Silica is heated with a concentrated (45%) solution of NaOH at473-523 K and 35- 36 bar pressure. Under these conditions, alumina dissolves as sodium metal aluminate and Silica as sodium silicate leaving behind the impurities.
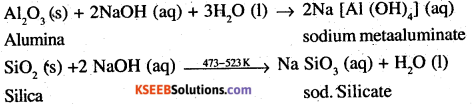
The resulting solution is filtered to remove the imdissol ved impurities, if any and neutralized by passing CO2 gas. Thereafter, the solution is seeded with freshly prepared samples of hydrated alumina when . hydrated alumina gets precipitated leaving sodium silicate in the solution.
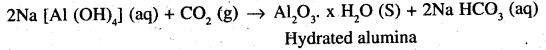
The hydrated alumina thus precipitated is filtered, dried and heated to give back pure Al2O3
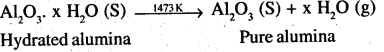
Alternatively, Serpeck’s process may be employed for the purification of white bauxite which contains Silica as the impurity.
The ore is powdered, mixed with coke and heated to 2073K in an atmosphere of N2 gas. Alumina combines with N2 to from aluminium nitride while Silica is reduced to silicon which volatilizes off at this temperature.
![]()
Aluminium nitride thus obtained is hydrolysed by water to form aluminium hydroxide which, when ignited, gives pure alumina.
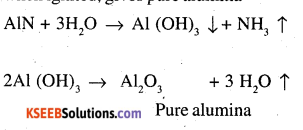
This process has one distinct advantage that ammonia is obtained as a by product.
Question 14.
Giving examples, differentiate between ‘roasting’ and ‘calcination’.
Answer:
The process of converting carbonates and hydroxide ores of metals to their respective oxides by heating them strongly below their melting points either in absence or limited supply of air is called calcination.
For example,
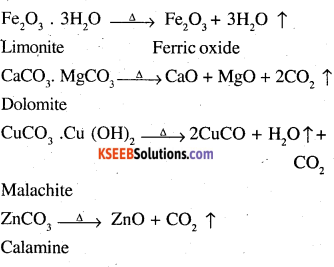
On the other hand, the process of converting a sulphide ore into its metallic oxide by heating strongly below its melting point in excess of air is called roasting. For example,
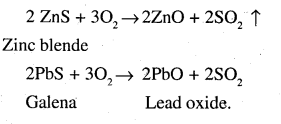
![]()
Question 15.
How is ‘cast iron’ different from ‘pig iron”?
Answer:
The iron obtained from blast furnace is called pig iron. It contains about 4% carbon and many other impurities (eg S, P, Si, Mn) in smaller amounts.
Cast iron, on the other hand, is made by melting pig iron with scrap iron and coke using hot air blast, it has slighly lower carbon content (about 3%) and is extremely hard and brittle.
Question 16.
Differentiate between “minerals” and “ores”.
Answer:
The naturally occurring chemical substances in form of which the metal occur in the earth along with impurities are called minerals. The minerals from which the metal can be extracted conveniently and economically is called an ore. Thus, all ores are minerals but all minerals are not ores. For example iron is found in the earth’s crust an oxides, carbonates and sulphides. Out of these minerals of iron, the oxides of iron are employed for extraction of the metal. Therefore, oxides of iron are the ores of iron. Similarly, aluminium occurs in earth’s crust in form of two minerals, i.e, bauxite (Al2O3. x H2O) and clay (Al2O3.2SiO. 2H2O). Out of these two minerals, Al can be conveniently and economically extracted from bauxite the ore of alluminium.
Question 17.
Why copper matte is put in silica lined converter?
Answer:
Copper matte chiefly consists of Cu2S along With some unchanged FeS. When a blast of hot air is passed through molten matte taken in a silica lined converter, FeS present is oxidised to FeO which combines with silica (SiO2) to form FeSiO3 slag.
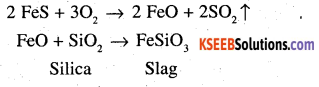
When whole of iron has been removed as slag, some of the Cu2S undergoes oxidation to form Cu20 which then reacts with more Cu2S to form copper metal.
2 Cu2S + 3O, → 2 Cu2O + 2SO2
2Cu2O + Cu2S → 6 Cu + SO2
Thus, copper matte is heated in Silica lined converter to remove FeS present in matte as FeSiO3 slag.
Question 18.
What is the role of cryolite in the metallurgy of aluminium?
Answer:
The role of cryolite is two- fold
- It make alumina a good conductor of electricity.
- It lowers the fusion (melting point) temperature of the both from 2323K to about 1140K.
Question 19.
How is leaching carried out in case of low grade copper ores?
Answer:
The leaching of the low grade copper ores is carried out with acids in presence of air when copper goes into solution as Cu2+ ions. Thus
2Cu (s) + 2H2SO4 (aq) + O2 (g) → 2CuSO4 (aq) + 2H2O(l)
or Cu (s) + 2H+ (aq) + \(\frac { 1 }{ 2 }\) O2 (g) → Cu2+ (aq) + H2O (l).
Question 20.
Why is zinc not extracted from zinc oxide through reduction using CO?
Answer:
The standard free energy of formation (Δf G°) of CO2 from CO (fig 6.8) is higher than that of the formation-of ZnO from Zn. Therefore, CO cannot be used to reduce ZnO to Zn.
Question 21.
The value of Δf G° for formation of Cr2 O3 is – 540 kjmol-1 and that of Al2 O3 is – 827 kjmol-1. Is the reduction of Cr2 O3 possible with Al ?
Answer:
The two equation are :
\(\frac { 4 }{ 3 }\) Al (s) + O2 (g) \(\frac { 2 }{ 3 }\)Al2O3 (S); ΔfG° Al,
Al2O3 = -827 kjmol-1 …………..(i)
\(\frac { 4 }{ 3 }\) Cr (s) + O2 (g) \(\frac { 2 }{ 3 }\)cr2O3 (s); ΔfG° = -827 kjmol-1 ……………(ii)
subtracting (ii) from (i), we have
\(\frac { 4 }{ 3 }\) Al (s) + \(\frac { 2 }{ 3 }\) Cr2O2 (s) → \(\frac { 2 }{ 3 }\)Al2O3(S)
+ \(\frac { 4 }{ 3 }\)cr(s): ΔfG° = -287kJmol-1
Since ΔfG° of the combined redox reaction is negative, therefore, reduction of Cr2O3by Al is possible.
Question 22.
Out of C and CO, which is a better reducing agent for ZnO ?
Answer:
The Δf G° of C02 from CO is always higher reduction of ZnO to Zn. In contrast, Δf G° of CO from C is lower at temperature above 1180K while that of CO2 from C is lower at temperatures above 1270K than Δf G° of ZnO. Thus above 1270K, ZnO can be reduced to Zn by C. In actual practice, reduction is usually carried out around 1673K. Thus out of C and CO, C is a better reducing agent than CO for ZnO.
![]()
Question 23.
The choice of a reducing agent in a particular case depends on thermodynamic factor. How far do you agree with this statement? Support your opinion with two examples.
Answer:
Thermodynamic factor helps us in choosing a suitable reducing agent for the reduction of a particular metal oxide to the metallic state as discussed below.
From the diagram, it is evident that metals for which the standard free energy of formation (∆f G°)of their oxides is more negative can reduce those metal oxides for which the standard free energy of formation (∆f G°)of their respective oxides is less negative. In other words. Any metal will reduce the oxides of other metals which lie above it in Ellingham diagram because the standard free energy change (∆f G °) of the combined redox reaction will be negative by an amount equal to the difference in (∆f G°) of the two-metal oxides. Thus, both Al and Zn can reduce FeO to Fe but Fe cannot reduce Al2O3 to Al and ZnO to Zn. Similarly, C can reduce ZnO to Zn but not CO.
Question 24.
Name the processes from which chlorine is obtained as a by-product. What will happen if an aqueous solution of NaCl is subjected to electrolysis?
Answer:
Sodium metal is prepared by Down’s process. It involves the electrolysis of a fused mixture -of NaCl and CaCl2 at 873K. During electrolysis, sodium is discharged at the cathode while Cl2 is obtained at the anode as a by product.
![]()
At cathode : Na+ (melt) + e– → Na (s)
At anode : Cl–(melt) → Cl (g) + e–
2Cl (g) → Cl2(g)
If, however, an aqueous solution of NaCl is electrolysed, is evolved at the cathode while Cl2 is obtained at the anode. The reason being that the E° of Na+/ Na redox couple is much lower (∈° = -2.71V) than that of H2O (∈°H2O/H2 = -0.83V) and hence water is reduced to H, in preference to Na+ ions. NaOH is however obtained in the solution.
At anode:
![]()
Cl– (aq) → Cl– (aq) + e–
2 Cl (g) → Cl2(g)
At cathode: 2H20(1) + 2e– → H2 (g) + 2OH– (aq)
Question 25
What is the role of graphite rod in the electrometallurgy of aluminium?
Answer:
In the electrometallurgy of aluminium, a fused mixture of alumina, cryolite and fluorspar (CaF2) is electrolysed using graphite as anode and graphite lined iron as cathode. During electrolysis, Al is liberated at the cathode while CO and CO2 are liberated at the anode.
At cathode Al3+ (melt) → A1 (1)
At anode : C (s) + O2- (melt) → CO (g) + 2e–
C (s) + 2O2- (melt) → CO2 (g) + 4e–
If instead of graphite, some metal is used as the anode, then O2 liberated will not only oxidised the metal of the electrode but would also convert some of the A1 liberated at the cathode back to Al2O3.
Since graphite is much cheaper than any metal, therefore, graphite is used as the anode. Thus the role of graphite is electrometallurgy of Al is to prevent the liberation of O2 at the anode which may otherwise oxidise some of the liberated Al back to Al2O3.
Question 26.
Outline the principles of refining of metals by the following methods:
(i) Zone refining
(ii) Electrolytic refining
(iii) Vapour phase refining
Answer:
(i)
1. This method is based on the principle that the impurities are more soluble in the molten state of metal (the melt) than in the solid state. In the process of zone refining, a circular mobile heater is fixed at one end of a rod of impure metal. As the heater moves the molten zone of the rod also moves with it. As a result pure metal crystallizes out of the melt and the impurities pass onto the adjacent molten zone. This process is repeated several times, which leads to segregation of impurities at one end of the rod. Then the end with impurities is cut off. Silicon, boron, gallium, indium etc can be purified by this process.
2. Column chromatography is a technique used to separated components of mixture where components are in minute quantities. In chromatography there are two phases : mobile phase and stationary phase. The stationary phase is immobile and immiscible. Al2O3 column is usually used as the stationary phase in column chromatography. The mobile phase may be a gas, liquid or super critical fluid in which the sample extracts dissolves. Then the mobile phase is forced to wave through stationary phase. The component that is more strongly absorbed on the column takes a longer time to travel than the component weakly , absorbed.
(ii) Electrolytic refining is the process of refining impure metal by using electricity. In this process impure metal is made the anode and a strip of pure metal is made the cathode. A solution of a soluble salt of the same metal is taken as the electrolyte. When an electric current is passed, metal ions from the electrolyte are deposited at the cathode as pure metal and the impure metal from the anode dissolves into the electrolyte in the form oftions. The impurities present in the impure metal gets collected below anode. This is called anode mud.
Anode : M → Mn++ ne–
Cathode : Mn+ + ne– → M.
(iii) Vapour phase refining is the process of refining metal by converting it into its volatile compound and then, decomposing is to obtain a pure metal to carry out this process.
(a) The metal should form a volatile compound with available reagent, and
(b) The volatile compound should be easily decomposable so that the metal can be easily recovered.
Nickel, Zirconium and titanium are refined using this method.
Question 27.
Predict conditions under which A1 might be expected to reduce MgO.
Answer:
Above 1350°C, the standard Gibbs free energy of formation of Al2O2 from Al is less than that at MgO from Mg. Therefore, above 1350°C, A1 can reduce MgO.
2nd PUC Chemistry General Principles and Processes of Isolation of Elements NCERT Additional Questions and Answers
Question 1.
State briefly the principles which serve as basis for the following operations in metallurgist.
(i) Froth flotation process
(ii) Zone refining ‘
(iii) Refining by liquation (Delhi 2008)
Answer:
(i) The principle of froth floatation is that the mineral particles are wetted by oils and gangues particles by water.
(ii) Zone refining is based on the principle that the impurities are more soluble in the melt than in the solid state of the metal
(iii) In refining by liquation method, a low melting metal like tin can be made to flow on sloping surface. In this way, it is separated from higher melting impurities.
![]()
Question 2.
What chemical principle is involved in choosing a reducing agent for getting the metal from its oxide ore? Consider the metal oxides, Al2O3 and Fe2O3 and justify the choice of reducing agent in each case.
Answer:
(i) Thermodynamic factor helps us in choosing a suitable reducing agent for the reduction of a particular metal oxide to the metallic state.
(ii) Any metal will reduce the oxides of other metals which lie above it in the Ellingham diagram because the standard free energy change (∆r Gθ) of the combined redox reaction will be negative by an amount equal to the difference in ∆r Gθ of two metal oxides. Thus both Al and Zn can reduce Fe2O3 to Fe but cannot reduce Al2O3 to Al.
3 (a) Name two alloys of copper.
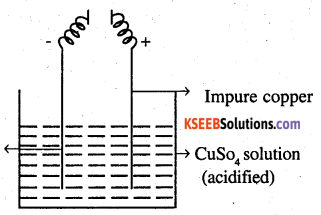
From the figure, give the electrode reaction. What is this process called?
Answer:
(a) Bras: Cu (60%) + Zn (40%)
Bronze: Cu (90%) + Sn (10%)
(b) This process is known as electrolyte refining. The impure metal is used as anode and pure metal is used as cathode. The electrolyte is a solution of a suitable salt of the metal. On passing electric current, the pure metal is deposited on the cathode. The solid impurities fall to the bottom of the cell and are recovered as anode mud. The soluble impurities go to the solution. For the purification of copper sulphate and the electrode reactions are
Anode: Cu → Cu2 + 2e–
Cathode: Cu2 + 2e– → Cu
Question 4.
Iron is extracted from its oxide ores
(i) Name two minerals of iron (Fe)
(ii) Do you think that all minerals of iron are used as ores of iron? Substantiate.
(iii) Name the purest form of iron.
(iv) How do you obtain the above form of iron?
Answer:
(i) Hematite (Fe2O3), magnetite (Fe3O4), siderite (FeCO3).
(ii) No. All minerals of iron are not used as ores of iron. Only those minerals from which the metals can be extracted economically are used as ores.
(iii) Wrought iron the purest form of iron.
(iv) Wrought iron is prepared by heating cast iron with hematite.
Question 5.
There are several methods for refining of crude metals
(a) For which metal purification is cupellation used for?
(b) Explain liquation.
(c) What is the principle of zone refining?
Answer:
(a) Copper.
(b) The impure metal is heated in the sloping floor. The low meting metal melts and flows down leaving behind the impurities. Thus method is known as liquation.
(c) This method is based on the principle that the impurities are more soluble in molten state. The impure metal, in the form of a rod, is melted over a very narrow region at one end. The impurities get accumulated in the molten region and the molten region is gradually transferred to one end by moving the heat source. The impurities collected in the molten region are swept at one end of the metal and are discarded.
Question 6.
(i) Ramu, one of your friends do not know the difference between a mineral and ore. How will you explain the above.
(ii) List out some metals which occur free in nature.
(iii) Also explain the terms gauge, Flux and slag in metallurgy.
Answer:
(i) The naturally occurring chemical substances in form of which the metal occur in the earth along with impurities are called minerals. The minerals from which the metal can be extracted conveniently and economically is called an ore. Thus, all ores are minerals but all minerals are not ores. For example iron is found in the earth’s crust an oxides, carbonates and sulphides. Out of these minerals of iron, the oxides of iron are employed for extraction of the metal. Therefore, oxides of iron are the ores of iron. Similarly, aluminium occurs in earth’s crust in form of two minerals, i.e, bauxite (Al2O3. x H2O) and clay (Al2O3.2SiO. 2H2O). Out of these two minerals, Al can be conveniently and economically extracted from bauxite the ore of alluminium.
(ii) Gold, silver and platinum.
(iii) Generally ores are contaminated with earthy, impurities and these impurities are called gangue or substance added is called flux. The flux combines with the impurities present in the ore to form an easily fusible material called slag.
![]()
Question 7.
While adding the raw materials into the blast furnace for the extraction of iron, it is forgotten to mix limestone with the charge.
(a) Predict the result of this mistake
(b) Give reason for this result.
(c) Bessemer converter follows blast furnace in steel industry. Justify.
Answer:
(a) Metal production and removal of impurities are not satisfactory.
(b) If limestone is not added, production of CO2 and CaO, become poor-CaO is a flux to remove acidic impurity
SiO2

(c) Blast Furnace produces pig iron and is converted to cast iron on remitting this cast iron is used in Bessemer converter to produce steel. So blast furnace is followed by a Bessemer converter in a steel industry.
Question 8.
Which of the ores mentioned in table 0.1 can be concentrated by magnetic separation method?
Answer:
Ores in which one of the components (either the impurity or the ore) is magnetic can be concentrated by magnetic separation method, e.g. Hematite (Fe2O2), magnetite (Fe3O4), siderite (FeCO3), iron pyrites (FeS) and copper pyrites (CuFeS2) can be concentrated by magnetic separation method.
Question 9.
St Significance of leaching in the extraction of aluminium?
Answer:
Leaching is used to remove insoluble impurities like silica, iron oxides etc. Alumina becomes soluble in NaOH while the impurities does not.
Question 10.
The reaction, Cr2O3+ 2Al → Al2O3 + 2Cr (Δ∈ P = -421KJ) is thermo dynamically feasible as is apparent from the Gibbs energy value. Why does it not take place at room temperature?
Answer:
Since ΔG is negative, the reaction is feasible. But certain amount of activation energy is required for this reaction to occur and hence it will not take place at room temperature (heating is required).
Question 11.
Is it true that under certain conditions, Mg can reduce Al2O3and Al can reduce MgO? What are those conditions?
Answer:
Yes from Ellingham diagram, it is found that the plots of A1 and Mg cross each other at 1350°C. Thus, below 1350°C can Mg reduce Al2O3 and above 1350°C A1 can reduce MgO.
![]()