Students can Download Karnataka SSLC Science Model Question Paper 2 with Answers, Karnataka SSLC Science Model Question Papers with Answers helps you to revise the complete Karnataka State Board Syllabus and score more marks in your examinations.
Karnataka State Syllabus SSLC Science Model Question Paper 2 With Answers
Time: 3 Hours
Max Marks: 80
I. Four alternatives are provided for each of the following questions or incomplete statements. Choose the most appropriate alternative and write with its alphabet. ( 8 × 1 = 8 )
Question 1.
Part of the flower that develops into fruit and part the seed that develops into root respectively are
A) Ovary and plumule
B) Plumule and radicle
C) Ovary and radicle
D) Ovary and ovule
Answer:
C) Ovary and radicle
Question 2.
The chemical equation that represent neutralization reaction among the following is
A) BaCl2 + H2SO4 → BaSO4 + 2HCl
B) MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
C) 2NaOH + H2SO4 → NaSO4 + 2H2O
D) AgNO3 + HCl → AgCl + HNO3
Answer:
C) 2NaOH + H2SO4 → NaSO4 + 2H2O
Question 3.
The action of bile can be termed as :
A) Esterification
B) Hydrogenation
C) Oxidation
D) Emulsification
Answer:
D) Emulsification
![]()
Question 4.
Which of the following are environment friendly practices ?
A) Carrying cloth – bags to put purchases while shopping.
B) Switching of unnecessary lights & fans
C) Walking to school instead of getting your mother to drop you on her scooter
D) All the above
Answer:
D) All the above
Question 5.
Bauxite is an ore of which metal
A) Iron
B) Aluminium
C) Copper
D) Tin
Answer:
B) Aluminium
Question 6.
The frequency of power supply used in India
A) 70 HZ
B) 60 HZ
C) 50 HZ
D) 30 HZ
Answer:
C) 50 HZ
Question 7.
On passing excess, of CO2 gas in an aqueous solution of calcium carbonate milkness of the solution
A) Persists
B) Fades
C) Deepens
D) Disappears
Answer:
B) Fades
![]()
Question 8.
Which of the following is a plant hormone
A) Thyroxin
B) Cytokinin
C) Insulin
D) Oestrogen
Answer:
B) Cytokinin
II. Answer the following questions ( 8 × 1 = 8 )
Question 9.
State ohm’s law.
Answer:
Under similar physical conditions and at constant temperature the current flowing thought a wire is directly proportional to the potential difference applied across its ends.
Question 10.
Why does a ray of light bend when it travels from one medium into another?
Answer:
Due to changes in velocity of light in the medium and to reduce the time to travel the same.
Question 11.
What id mutation.
Answer:
ways to protect their DNA from changes inspite of those mechanisms, however changes in the DNA occasionally do occur. Any changes in the DNA sequence is called a mutation.
Question 12.
Why does raw bread taste sweeter on mastication ?
Answer:
Salivary amylase which converts starch into sugars.
![]()
Question 13.
Why does a copper vessel develop with green coating in rainy season ?
Answer:
Oxygen, carbon dioxide and water vapour of the air attack copper metal forming green coloured basic copper carbonate. The formula is CuCO3, CU(OH)2
Question 14.
Will Current flow more easily through a thick wire or thin wire of the same material when connected to the same source ? Why ?
Answer:
The current will flow more easily through thick wire because the resistance of a conductor is inversely proportional to area of cross section. If thickness of the wire less is resistances and hence more easily the current flows.
Question 15.
Why does distilled water not conduct electricity whereas rain water does ?
Answer:
Distilled water cannot conduct electricity because it does not contain ions while rainwater conducts electricity as it contains ions due to presence of dissolved salts in it.
Question 16.
What is reflex arc ?
Answer:
The structural and functional unit that carries out reflex action is called a reflex arc.
III. Answer the following questions ( 8 × 2 = 16 )
Question 17.
An electric bulb of 200 Ω draws a current of 1 ampere. Calculate the power of the bulb. The potential difference at is ends and the energy in kwh consumed in burning it for 5h.
Answer:
Power of the bulb
Power of the bulb P = I2R= (1)2 x 200
P = 200 W
Energy consumed by bulb in 5h in burning
= Power x time
= 200 x 5
= 1000 wh = 1 kwh.
![]()
Question 18.
What are structural isomers ? Name the first member of alkanes that shows structural isomerism.
Answer:
Compounds with identical molecular formula but different structures are called structural isomers.
Butane or C4H10
Question 19.
Draw the circuit symbols of the following
i) Variable resistance
ii) Wire crossing without joining.
Answer:
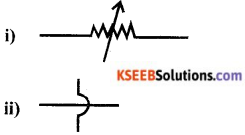
Question 20.
Describe how hydro-energy can be converted into electrical energy.
Answer:
- High rise dams are constructed on the river to obstruct the flow of water to collect it at suitable height. The stored water has a lot of potential energy.
- The water from a suitable height is allowed to fall on the blades of a turbine located at the bottom of a dam through a pipe.
- Kinetic energy of flowing water rotates the turbine helping the armature coil of generator to rotate rapidly in the magnetic field. Thus hydroelectricity is generated.
OR
Explain how the tidal energy is harnessed and write one limitation of the use of tidal energy.
Answer:
Tidal energy is harnessed by constructing a dam near the shore. During the high tidal water flows into the dam during the low tides later flows out this flowing water rotates the turbine present at the opening of the dam and produces electricity.
![]()
Question 21.
Draw a diagram of excretory system in human beings label the following parts.
i) Urethra ii) Left renal vein
Answer:
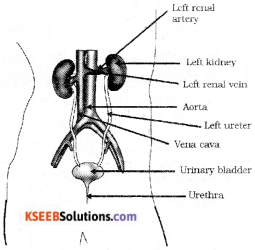
Question 22.
Draw the ray diagram showing the position of the object and image to get real the inverted image, whose size is same as the object using convex lens.
Answer:
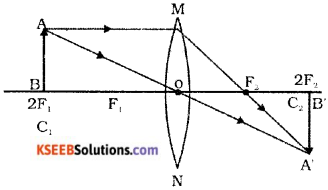
Question 23.
Why must we conserve our forests ? List any two causes for deforestation to take place.
Answer:
We must conserve our forests as they are of great value the reasons for conserving forests are.
- Forests help in protection of land and retaining sub soil water.
- Forests check floods and maintain ecosystem.
- Therefore forests must be conserved for economic and social growth.
Two causes of deforestation taking place are.
- For industrial needs.
- For development projects like building of road or dams.
![]()
Question 24.
Give reasons :
i) “Alloys of iron-are more useful when compared to pure iron”
Answer:
- Pure iron is very soft
- Stretches, when easily when hot
- Alloys are hard
- The properties of iron can be changed if it is mixed with other substances.
ii) Aluminium oxide is called amphoteric oxide
Answer:
Aluminium oxide (Al2O3) reacts with both acids as well as bases to produce salt and water.
OR
i) Metals are regarded as electro positive elements
ii) Articles of aluminium do not corrode even though aluminium is an active metal.
Answer:
i) Metals have a tendency to lose one or more electrons to form positive ions, therefore metals are called electro positive elements.
ii) Aluminium being a reactive metal readily combines with oxygen to give a protective layer of Al2O3. As a result further corrosion stops.
IV. Answer the following questions. ( 9 × 3 = 27 )
Question 25.
a) Which disease is caused in human beings due to depletion of ozone layers in the atmorphere.
Answer:
Skin cancer is caused in human beings due to the depletion of ozone layer in the atmosphere.
b) What step is being taken to limit the damage to the ozone layer.
Answer:
- Judicious use of aerosol spray propellants such as fluro carbons and chlorofluro- carbons which cause depeletion or hole in ozone layer.
- Control over large scale nuclear explosions and limited use of supersonic planes.
![]()
Question 26.
a) State the modern periodic law.
Answer:
“Properties of elements are the periodic function of their atomic number”
b) How does the electronic configuration of atom of an element relate to its position in the modren periodic table? Explain with one example.
Answer:
The position of elements depend upon number of valence electrons which depend upon electronic configuration those elements which have same valence electrons occupy same group. Those elements which have one valence electrons belong to group 1 elements, which have two valence electrons belong to group 2. Period numbers is equal to the number of shell.
Example : atomic number of sodium (Na) is 11, so electronic configuration will be 2, 8, 1. Sodium has one valence electron in valence shell so it belong to group 1 as sodium has three shells, so it belong to 3rd period.
OR
a) How does the electronic configuration of atom relates to its position in the modern periodic table.
Answer:
In the periodic table elements are placed according to their atomic number. If an element has only one shell in its electronic configuration it is placed in the first period, if the element has two shells then it is placed in the second period and so on. Vertical column in the periodic table are called groups there are eighteen groups and in a group all the elements have same number of valence electron.
b) Would you place the two isotopes of chlorine Cl – 35 and Cl – 37, in different slots because of their different atomic masses are in the same slot because their chemical properties are the same. Justify your answer.
Answer:
No. Cl – 35 and Cl – 37 will be placed in same slot. The modem periodic table is based on atomic numbers not atomic mass since, both Cl – 35 and Cl – 37 have same atomic numbers hence they are placed in same slot.
![]()
Question 27.
What is meant by electric current? Name and define its SI unit. In a conductor electrons are flowing from B to A. What is the direction of conventional current? Give justification for your answer.
Answer:
A steady current of 1 ampere flows through a conductor. Calculate the number of electrons that flows through any section of the conductor is 1 second (charge on electron 1.6 x 1019 Coulomb)
Electric current : The amount of charge Q flowing through a particular area of cross section in unit time ‘t’ is called electric current, ie., Electrict current, I = Q/t
SI unit of electric current is ampere
One ampere of current is that current which flow when one coulomb of electric charge flowing through a particular area of cross section of the conductor is one second, i.e, 1A = 1 CS-1
The direction of conventional current is A to B, ie., opposite to the direction of flow of electrons. In a metal, flow of electrons carrying negative charge constitutes the current. Direction of flow of electrons given the direction of electronic current by convention, the direction of flow of positive charge is taken as the direction of conventional current charge
= q = ne
For q = I Coulomb
![]()
OR
a) Write two points of difference between electric energy and electric power
b) Out of 60 W and 40 W lamps which one has higher electrical resistance when in use.
c) What is the commercial unit of electric energy? Convert it into joules. Electric energy
Answer:
The work done or energy supplied by the source in maintaining the flow of electric current is called electrical energy. It appears in the from of heat given by
H = VIt = \(\frac{v^{2} t}{R}\) = I2RT
- It is equal to be product of power and time E = P × t
- It SI unit is joule (J)
1J= 1W × 1S
Electric power:
The time rate at which electronic energy is consumed or dissptied by an electrical device is called electric power and is given by
P = VI = \(\frac{v^{2}}{R}\) = I2R
It is equal to the rate of doing work by an energy source
P = \(\frac{\mathrm{W}}{\mathrm{t}}\)
Its SI unit is watt(W)
1W = 1 JS-1
b) For the same applied voltage P & \(\frac{1}{\mathrm{R}}\)
i,e less the same power of electrical device higher it is electrical resistance.
c) Kilowatt hour – commercial unit of electrical energy.
1 kwh = 1000 wh = 1000 J/S × 3600 see
= 3600000 J = 3.6 × 106J
![]()
Question 28.
Draw the diagram of the arrangement of apparatus showing the reaction of zinc granules with dilute sulphuric acid and testing hydrogen gas by burning and label the soap bubbles filled with hydrogen.
i) Soap solution ii) Delivery tube
Answer:
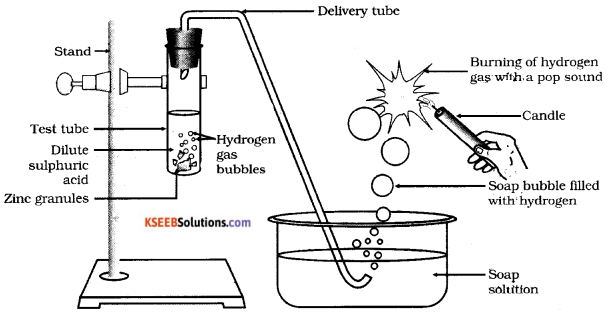
Question 29.
What is Tyndall effect ? What is its cause? Explain two phenomenon observed in daily life which are based on Tyndall effect.
Answer:
Tyndal effect : When a beam of light is passed through a colloidal solution placed in a dark room the path of beam becomes illuminated when observed through a microscope placed perpendicular to the path of light. This effect is called Tyndall effect.
Causes of tyndal effect : The size of the collidal partical is relatively larger then the solute particals of a true solution. The collidal particles first absorb energy from the incident light and then a part of energy from their surface. Thus tyndall effect is due to scattering of light by the colloidal particals and the colloidal particles are seen to be moving as points of lights moving againist a dark background.
Daily life examples :
- When sunlight passes though a canopy of a dense forest the tiny water droplets in the mist scatter light and become visible.
- When a fine beam of sunlight enters a smoke filled room through a small hole the smoke particals become visible due to the scattering of light.
OR
a) What is myopia ? State the two causes of myopia
Answer:
Myopic eye can see objects at short distance inability of the eye in viewing long distant objects the image falls before retina causes of myopia.
- Elongation of eye ball
- Excessive curvature in cornea.
b) Why is the normal eye unable to focus on an object placed whith in 10 cm from the eye?
Answer:
A normal eye is unable to clearly see the objects placed closer then 25 cm because the ciliary muscles of eyes are unable to contract beyond a certain limit.
When the object is placed at a distance less or more than 25 cm from the eye than the object appears blurred and induces strain in the eyes.
Question 30.
Draw a diagram of human brain label the parts.
i) Cranium ii) Pituitary gland
Answer:
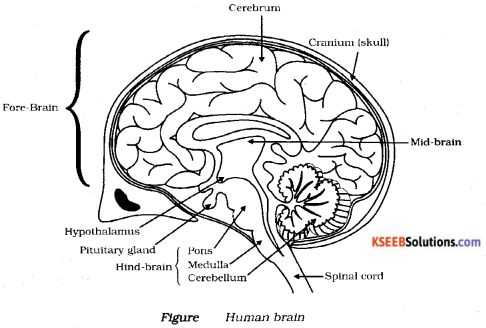
![]()
Question 31.
a) State in brief the function of the following organs in the human female reproductive system. Ovary’, fallopian tube, uterus
Answer:
Function of ovary :
- Production of female gamete.
- Production of female hormone
Function of fallopian tube :
- Site of fertilization
- Transfer of female garnets from ovary
Function of uterus:
- Implantation of zygote fertilizes the egg.
- Nourishment to the developing embryo
b) Why mensuration take place ?
Answer:
Menstruation : It is the periodic breakdown of uterine lining and it is removed along with blood & mucous in human female uterine lining is required to nourish the embryo that is formed if fertilization takes place. In absence of fertilization the lining is not required and hence is shed in the from of menstruation.
Question 32.
a) Name two metals which can evolve hydrogen gas by reacting with very dilute nitric acid.
b) Explain the formation of MgO by transfer of Electrons.
Answer:
a) Magnesium and Manganese
b) Formation loses two electrons , and forms
Magnesium ions to complete its octet.
Mg → Mg2+ + 2e-
oxygen gains that two electrons to become O2-
Mg2+ + O2 → MgO
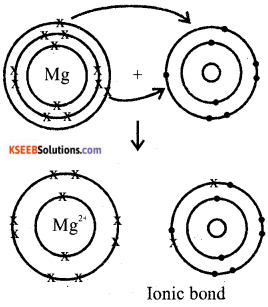
Question 33.
Define genetics what is the contribution of Mendel in the field of genetics ?
Answer:
The branch of biology that deals with the study of heredity and variation is called genetics Gregor Johann Mendel was the first person to carry out experiment regarding the heredity of certain characters from one generation to another in a scientific manner. He worked mainly on the garden pea plant.
His observations regarding the occurance of contrasting characters in various generations of garden pea led him to interpret that these are controlled by units which he called factors. These factors are today know as genes. He is also know as the ‘father of genetics’.
OR
a) How can we trace evolutionary relationships ?
Answer:
Evolutionary relationships can be traced by studying fossils, by studying homologous and analogous organs, by comparing the embryo or of different animals and by comparing the DNA’s of different species.
b) Define variation in relation to a species. Why is variation beneficial to the species.
Answer:
Variation are differences that occur between the organisms of same species in spite of the same basic features.
Variation in species promotes survival of an organism in the changing environment by increasing the adaptability.
V. Answer the following questions. ( 4 × 4 = 16 )
Question 34.
a) Explain why the planets do not twinkle ?
Answer:
Planets are much closer to the earth and are seen as extended sources, so a planet may be considered as a collection of a large number of point size light sources, although light coming from individual point sized sources flicks but the total amount of light entering our eye from all the individual point sized sources average out to be constant thereby planets appear equally bright and there is no twinkling of planets.
b) What is the colour of the clear sky during day time? Give the reason for it.
Answer:
Clear sky appears blue when sunlight passes through the atmosphere having the molecules of air and other fine particles whose size is smaller then the wavelength of visible light. These molecules and particular scatter the blue colour more strongly then the other colours of spectrum. As the wave length of blue colour is more, this scattered blue light enters our eye, so, the colour of sky appears blue to us during day time.
![]()
Question 35.
What is an esterification reaction ?
Answer:
When carbonic acid reacts with alcohol in the presence of cone. H2SO4, pleasant fruity smelling compound is formed this type of reaction is called esterification reaction.
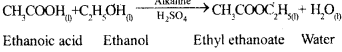
b) Write a chemical equation in each case to represent -the following types of chemical reaction of organic compounds :
i) Oxidation reaction
ii) Addition reaction
iii) Substitution reaction.
Answer:
i) Oxidation reaction :

ii) Addition reaction :
![]()
iii) Substitution reaction :
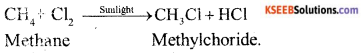
OR
a) What is meant by saponification? Give an explanation.
Answer:
It is the reaction that forms soap when an ester reacts with water in the presence of base a salt of carboxylic acid and an alcohol are produced. Such a reaction is called saponification.
For example r When ethyl ethanote is heated with a solution of sodium hydroxide sodium ethanote and ethanol are produced.
CH3COO C2H5 + NaOH → CH3COONa + C2H5OH
b) Draw the structure of the following compound and identify functional group present in them, i) Butanoic acid ii) Bromopropane.
Answer:
i) Function group is carboxylic acid
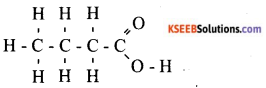
ii) Function group is Bromine
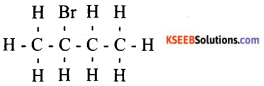
Question 36.
How are fats digested in our bodies ? Where does this process take place ? Explain.
Answer:
Digestion of fats takes place in the small intestine the fats are digested by the digestive enzymes. The fats are present in the from of large globle in the small intestine. Bile juice secreted by the liver is produced in the intestine along with pancreatic juice. The bile salts present in the bile juice emulsify the large globles of fats.
So by emulsification large globles breakdown into fine globules to provide large surface area to act upon by the enzymes. Enzyme present in the pancratic juice cause breakdown of emulsified fats. Glands present in the wall of small intestine secretes intestinal juice which contains lipase enzyme that converts fats into fatty acids and glycerol.
![]()
Question 37.
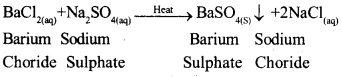
a) What do you mean by a precipitation reaction? Explain by giving example.
b) Explain the following in terms of gain or loss of oxygen with examples.
Answer:
a) Any reaction in with an insoluble solid is produced is called a precipitate reaction. For example:
b) Oxidation : It is defined as process which involves gain of oxygen
For example :
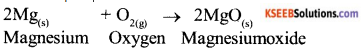
Reduction : It is defined al process which involves loss of oxygen.
For example :
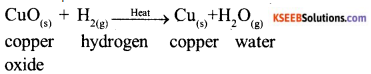
![]()
VI. Answer the following question. ( 1 × 5 = 5 )
Question 38.
What are magnetic field lines ? How is the direction of a magnetic field at point determined? Mention two important properties of the magnetic field lines.
Answer:
The space surrounding a magnet in which magnetic force is exerted is called a magnetic filed. Magnetic field lines are the lines that are drawn at every point indicating the direction in which a north pole would move if placed at that point. They are determined by placing an imaginary hypothetical north pole at that point and finding the direction in which it would move due to the magnetic filed at that point of a compass needle gets deflected when placed near a magnet due to the magnetic force exerted by the magnet on it.
Some important properties of magnetic field lines are :
- The tangent drawn at any point on the field line indicates the direction in which a north pole would move if placed at that point
- The relative strength of the field is proportional to the degree of closeness of the lines. The more clustered they are the stronger the field in the region.
- The magnetic field lines never intersect. This is because a pole can move only in zone direction and if the lines intersect they would have to move in two direction simultaneously which is impossible.