Students can Download Karnataka SSLC Science Model Question Paper 4 with Answers, Karnataka SSLC Science Model Question Papers with Answers helps you to revise the complete Karnataka State Board Syllabus and score more marks in your examinations.
Karnataka State Syllabus SSLC Science Model Question Paper 4 With Answers
Time: 3 Hours
Max Marks: 80
I. Four alternatives are provided for each of the following questions or incomplete statements. Choose the most appropriate alternative and write with its alphabet. ( 8 × 1 = 8 )
Question 1.
The organ which perform different function but have the same basic structure are called.
A) Vestigal organ
B) Analogous organs
C) Homologous organs
D) Analytic organs
Answer:
C) Homologous organs
Question 2.
Identify the correct statement among the following with respect to plant hormones.
A) Cytoldnin promotes wilting of leaves.
B) Auxin inhibits stem elongation
C) Abscisic acid inhibits growth of plants
D) Gibberillin promotes falling of leaves.
Answer:
C) Abscisic acid inhibits growth of plants
![]()
Question 3.
The watershed management
A) Increases drought and floods
B) Increases production and income of the watershed community
c) Decreases the biodiversity of the downstream reservoirs.
d) increases deforestation
Answer:
B) Increases production and income of the watershed community.
Question 4.
pH of ammonium chloride (NH4Cl) or copper sulphate (CuSO4) solution be
A) 7
B) >7
C) <7
D) 0
Answer:
C) <7
Question 5.
Which of the following has a triple bond.
A)C2H6
B) C3H4
C)C3H8
D) C3H6
Answer:
B) C3H4
Question 6.
Characters transmitted from parents to offspring are present in
A) Cytoplasm
B) Ribosomers
C) Golgi complex
D) Genes
Answer:
D) Genes
![]()
Question 7.
What is that instrument which can detect the presence of electric current in a circuit.
a) Galvanometer
b) Motor
c) Generator
d) None of above
Answer:
a) Galvanometer
Question 8.
Aluminium is used for making cooking utensils. Which of the following properties of aluminium are responsible for the same
i) Good thermal conductivity
ii) Good electrical conductivity
iii) Ductility
iv) High melting point.
A) (i) and(ii)
B) (i) and (iii)
C) (ii) and (iii)
D) (i) and (iv)
Answer:
D) (i) and (iv)
II. Answer the following questions ( 8 × 1 = 8 )
Question 9.
Meat is easier to digest as compared to grass. Why ?
Answer:
It is easier to digest meat because our digestive juices contain enzymes which can easily digest meat but our body does not digest cellulose which is main component of grass.
Question 10.
Define accommodation of an eye ?
Answer:
The ability of the eye to focus both near and distant objects by adjusting the focal length is called accommodation of the eye.
![]()
Question 11.
What happens if fallopian tube is blocked ?
Answer:
If fallopian tube is blocked the egg will not be able to reach the uterus in such case fertilization will not take place.
Question 12.
Convex mirror is commonly used as rear view mirror in vehicles. Why ?
Answer:
- They always give an erect diminished image.
- Also they have a wider field of view as they are curved outwards.
Question 13.
What is meant by the statement that the rating of fuse in a circuit is 5A.
Answer:
It means maximum current of 5A can pass through the fuse without melting it.
Question 14.
List any two causes for the failure of sustained availability of ground water
Answer:
- Loss of vegetation cover
- Pollution from industrial effluents
Question 15.
What is the role of the seminal vesicles and the prostate gland.
Answer:
The secretion from seminal vesicles and prostate glands lubricate the sperms and provide a fluid medium for easy transport the secretions also provide nutrient in the from of fructose calcium and some enzymes.
![]()
Question 16.
What happens when magnesium ribbon burns in air ?
Answer:
When magnesium ribbon bums in air it combines with the oxygen to from magnesium oxide.
2Mg(s) + O2(g) → 2Mgo(s)
III. Answer the following questions ( 8 × 2 = 16 )
Question 17.
An ammeter is always connected in series across a circuit element what happens when it is connected in parallel with a circuit element.
Answer:
An ammeter is law resistance device when it is connected in parallel the resistance of the circuit reduces consider blu therefore a large current flows through the circuit by virtue of circuit element may damage.
OR
a) Define electric power express it in terms of potential difference V and resistance R.
b) What is meant by saying that the potential difference between two points is IV?
Answer:
a) Electric power : it is the rate of doing work by an energy source or the rate at which the electrical energy is dissipated or consumed per unit time in the electric circuit is called electric power.
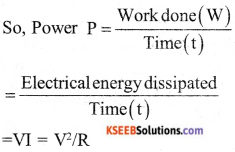
b) If 1 I of work is required to move a charge of amount 1C. from one point to another then it is said that the potential difference between the two points is 1V.
Question 18.
Draw a diagram of an excretory system in human beings.
Answer:
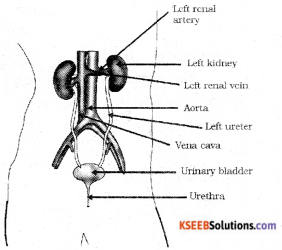
![]()
Question 19.
Explain the cause of shoots of the plants bending towards light.
Answer:
Stems are positively phototropic and bend towards the direction of light the movement is due to occurrence of more auxin on the darken side and lesser auxin on the illuminated side as a result there in more growth on the darker side which causes the stem to bend towards light.
Question 20.
Draw the diagram of arrangement of apparatus used to show the reaction of zinc granules with dilute sulphuric acid and testing hydrogen gas by burning. Label the following parts.
1) Soap solution ii) Delivery tube
Answer:
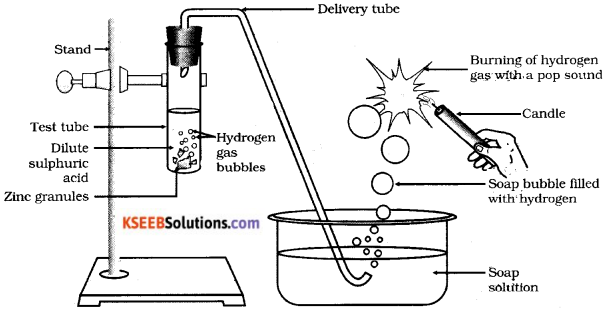
Question 21.
Variations that confer an advantage to an individual organism only will survive in population. Justify.
Answer:
Variations is the difference in the characters or traits among the individuals of a species sexual reproduction of organisms produces variation. The variations produced in organisms during successive generations gets accumulated in the organism the significance of variations shows up only if it continues to be inherited by the offspring for several generation.
Question 22.
List the three kinds of blood vessels of human circulatory system and write their functions.
Answer:
- Arteries : They carry blood away from the heart to various organs of the body.
- Veins : They collect the blood from different organ and bring it back to the heart.
- Capillaries : Exchanges of material . between the blood and surrounding cells take place across the thin walls of capillaries.
OR
Why is necessary to separate oxygenated and deoxygenated blood by mammals and birds?
Answer:
Mammals and bird are warm blooded animal they constantly use energy to maintain their body temperature they have higher energy needs and so they require more oxygen to produce energy. Thus it is important that their oxygenated blood does not get mixed up with deoxygenated blood.
Question 23.
Draw the diagram of an electric generator and label the following parts.
i) Armature ii) Rings
Answer:
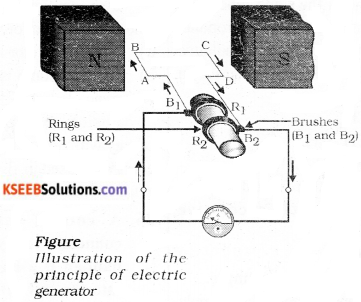
![]()
Question 24.
What is the function of receptors in our body ?
Answer:
Think of a situation where receptors do not work properly what problems are likely to arise.
Function of receptors:
- They sense the external stimuli such as heat or pain
- They also trigger an impulse in the sensory neuron which sends message to the spinal cord.
When the receptors are damaged the external stimuli transferring signal to the brain are not felt. For example in the case of damaged receptors if we accidentally touch any hot objects we cannot feel the pain or heat. Receptors cannot precive the external stimuli of heat and pain.
IV. Answer the following questions ( 9 × 3 = 27 )
Question 25.
Mention why is it not possible to make use of solar cells to meet all our energy needs ?
Answer:
State three reasons to support your answer, also mention three uses of solar cell.
- It is not possible to make use of solar cells to melt all our energy needs because.
- Limited availability of special grade semi conducting materials such as silicon and germanium.
- Solar cell have lower efficiency as they depend entirely on intensity of solar radiation.
- The process of manufacturing of solar cells is very expensive silver used for inter connection of cell in the panel further add to the cost.
Uses of solar cell:
- They provide electric power to satellites and space probes.
- They provide electric power to offshore drilling plants from light houses.
- The T.V relay stations located in remate area use solar panels to get electric power for wireless transmission.
OR
Compare and contrast fossil fuel and the sun as direct sources of energy ?
Answer:
Fossil fuel are energy sources such as coal and petroleum obtained from underneath of earth’s crust they are directly available to human being for use hence fossil fuels are the direct source of energy these are limited in amount these are non renewable sources of energy because three cannot be replenished in nature fossil fuel-take million of years for their formation.
If the present fossil fuel of the earth gets exhausted its formations will take several years, fossil fulls are also very costly.
On the other hand solar energy is a renewable and direct source of energy and will do so for the next five billion years, solar energy is available free of cost to all in unlimited amount replenishes in the sun itself.
Question 26.
Draw the ray diagram showing the image formation by a convex lens, when the object is at principal focus fl. with the help of the diagram mention the nature of the image formed.
Answer:
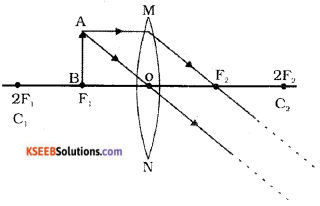
The nature of the image is real and inverted.
Question 27.
Explain the breakdown of glucose in aerobic respiration and anaerobic respiration.
Answer:
i) In the presence of oxygen : When breakdown of glucose is carried out in the presence of oxygen in a cell, it is called as aerobic respiration. Glucose is converted into a 3 carbon molecule called pyruvate which further breakdown in the presence of oxygen to form carbon dioxide and water. Energy is released in this process.
![]()
ii) In the absence of oxygen : When breakdown of glucose is carried out in the absence of oxygen in a cell. It is called as anaerobic respiration. This process is called as fermentation in microbes. Ethyl alcohol or lactic acid is produced by the breakdown of pyruvate
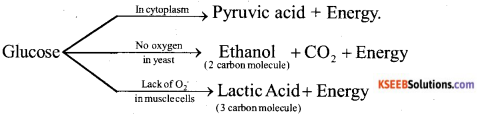
OR
Explain the process of transportation of substances in phloem.
Answer:
Trans-location, i.e., transportation of food , in plants takes place through phloem. Sieve tubes with the help of adjacent companion cells in the phloem move the soluble food in the form of sucrose, amino acids etc in both upward and downward directions. Live cells of phloem actively take up material like sucrose using energy from ATP. This increases the osmotic pressure of the tissue causing water to move into it.
This pressure moves the material in the phloem to tissues which have less pressure. According to the needs of the plants food from leaves to storage organs of roots, fruits and seeds and the growing regions or from stored parts such as roots or stem to the buds (in Spring) which needs energy to grow is transported.
Question 28.
a) Why do stars twinkle
Answer:
Stars twinkle due to atmospheric refraction of starlight as the stars are very far away. They behave as almost point sources of light as on account of atmospheric refraction. The path rays of light coming from the star goes on varying slightly the apparent position of the star fluctuates and the amount of starlight entering the eye flickers. So sometimes the star appears brighter and at some other time fainter, thus the stars twinkle.
b) State two properties of the image formed by the eye lens on the retina.
Answer:
- Image on the retina is real and inverted.
- Diminished in size.
![]()
Question 29.
Draw a neat diagram of action of steam on a metal label the following parts,
i) Metal sample
ii) Delivery tube.
Answer:
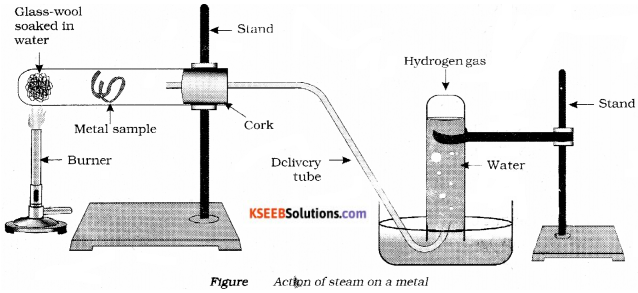
Question 30.
i) What is genetics ?
Answer:
Science which deals with the study of heridity and variations is called genetic.
ii) Give the common name of a plant on which Mendel performed its experiments.
Answer:
Pea plant.
iii) What for did Mendel use the term factors and what are these factors called now ?
Answer:
Mendel used the term factors for genes.
iv) What are genes ? Where are the genes located?
Answer:
Genes is the unit of inheritance it is a part of the chromosome which controls the appearance of a set of heredity character gender are located on the chromosomes.
OR
a) Define evalution? Describe the contribution of landmark.
Answer:
Evalution is referred to as the changes acquired by a species or a certain population of a species gradually over a long period of time these changes should be heritable contribution of landmark.
According to the theory of inheritance of acquired characters or Lamarkism, put forward by lamark. The use and disuse of an organ leads to acquiring change in the features of that organ.
these change are also inherited by the offsprings the favorable variations caused due to use and disue after a considerably long period of time result in evolution of a new species.
b) How do homologous organs provide evidence in support of evolution ?
Answer:
The presence of homologous organs indicates that all vertebrates have a common ancestry. Similarly all organs and system of the vertebrates show the fundamental similarities Which point towards common ancestry.
Question 31.
a) What are trophic levels ?
Answer:
Trophic levels are the feeding level in an ecosystem, the trophic level of living beings represent their placement in a food chain it also tell the order of consumption and energy transfer throughout the ecosystem.
b) What will happen if we kill all organisms in one tropic level.
Answer:
If we kill all the organisms in one trophic level the following effects will taken place
- The population of organisms in previous tropic level will increase.
- The organisms in next trophic level will not be able to get the food so they will migrate to some other ecosystem or die.
- It will cause an ecological imbalance in the food chain.
![]()
Question 32.
a) State the principle of an electric generator
Answer:
An electric generation is based on the principle of electromagnetic induction when a rectangular coil is rotated in a uniform magnetic field, an induced voltage is generated between the ends of the coil.
b) Write the difference between direct current and alternating current.
Answer:
Direct current:
- Its magnitude is constant and flows in one direction only.
- The frequency of DC is zero
Alternating current:
- Its magnitude and direction reserves periodically.
- The frequency of AC is finite.
Question 33.
a) What is a universal indicator ?
Answer:
A universal indicator is a mixture of a number of indicators which shows different colors at different pH values.
b) A milk man adds a very small amount of baking soda to fresh milk.
i) Why does he shift the pH of the fresh milk from 6 to slightly alkaline.
Answer:
Since the fresh milk is acidic it turns sour early in presence of baking soda, milk become alkaline and does not turn sour easily because of alkali does not allow the milk to become more acidic early.
ii) Why does this milk take a long time to set as curd.
Answer:
When the milk sets to curd the pH decreases i.e., it becomes more acidic the presence of alkali does not allow it to become more acidic easily hence it will take a long time to set as curd.
OR
a) Why does an aqueous solution of an acid conduct electricity.
Answer:
The presence of hydrogen (H+) or hydronium(H3O+) ions in the aqueous solution of an acid are responsible for conducting electricity.
b) How is plaster of paris prepared ? What reaction takes plaster place when its sets to a hard mass.
Answer:
Plaster of Paris is prepared by heating gypsum to a temperature of 100°C the following reaction takes place :
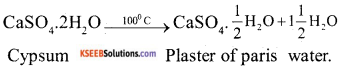
On mixing with water it converts to a hard mass to form gypsum again i,e the reverse reaction takes place.
![]()
V. Answer the following questions. ( 4 × 4 = 16 )
Question 34.
When we say the resistors are in parallel ?
Write the expression for the resistors in parallel. How many 176 Ω resistors (in parallel) are required to carry on a 220 V line ?
Answer:
We can say that resistors are in parallel if same potential difference is applied across them.
For three resistors if we connect them in parallel the equivalent resistance is R
\(\frac{1}{R}=\frac{1}{R_{1}}+\frac{1}{R_{2}}+\frac{1}{R_{3}}\)
For x number of resistors of resistance 176 Ω the equivalent resistance of the resistors connected in parallel is given by ohm’s law as
V = IR
R = \(\frac{\mathrm{V}}{\mathrm{I}}\)
Where,supply voltage, V = 220 V
current I = 5A
Equivalent resistance of the combination = R given as

Question 35.
a) List any three observation that determine that a chemical reaction has taken place. Also list three in formation that cannot be obtained about a chemical reaction merely by its chemical equation.
b) Balance the following chemical equations
i) Fe + H2O → Fe3O4 + H2
ii) CO2 + H2O → C6H12O6 + O2
Answer:
The three observations are
- Change of colour
- Change of temperature
- Evolution of gas
The three information are
- Atmospheric conditions
- catalyst iaoived
- Physical state of reactants and products
Balancing equations
- 3Fe+4H2O → Fe3O4 + 4H2
- 6CO2 + 6H2O → C6H12O6 + 6O2
OR
a) Explain the following in terms of gain or lose of oxygen with two examples each.
i) Oxidation ii) reduction.
Answer:
i) Oxidation : It is defined as process which involves gain of oxygen for example
![]()
ii) Reduction: It is defined as the process which involves loss of oxygen for example
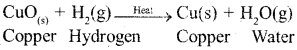
b) Balance the following chemical equations.
i) HNO2+Ca(OH)2 → Ca(NO3)2 +H2O
ii) NaOH+ H2SO4 → Na2SO4+H2O
Answer:
i) 2HNO3(aq) + Ca(OH)2(aq) → Ca(NO3)2(aq) + 2H2O
ii) 2 NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + 2H2O (I)
![]()
Question 36.
a) State Mendeleev’s periodic law.
b) Did Mendeleev have gaps in his periodic table?
c) Any three limitations of Mendeleev’s classification.
d) Does electronic configurations of atoms change in a period with increase in atomic number?
Answer:
a) Mendeleev’s periodic law states that “the physical and chemical properties of all elements are the periodic function of their atomic masses”.
b) Gaps were left for undiscovered element in the Mendeleev’s periodic table.
c) i) Position of hydrogen was not justified
ii) Increasing order of atomic mass could not be maintained
iii) Isotopes have similar chemical properties but different atomic masses, they cannot be given separate places.
d) Number of shells remain the same, number of valence electrons goes on increasing from left to right in a period till octet is complete.
Question 37.
a) State the two laws of reflection of light.
b) The refractive induces of four media A, B, C & D are given in the following table.
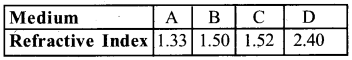
If light travels from one medium to another in which case the change in speed will be
i) minimum ii) maximum
Answer:
a) Laws of reflection of light are :
- The angle of incidence is equal to the angle of refection.
- The incident ray the normal to the reflecting surface at the point of incidence and reflected ray from that point all lies in the same plane.
b)i) Minimum change is seen as light moves between 1.50 and 1.52 i,e B and
ii) Maximum change when light moves between 1.33 and 2.40 i,e A and D
![]()
VI. Answer the following question. ( 1 × 5 = 5 )
Question 38.
a) What is hydrogenation? What is its industrial application.
Answer:
Addition of hydrogen to unsaturated hydrocarbons in presence of a catalyst such as nickel or palladium to form saturated hydrocarbons is called hydrogenation For Example:
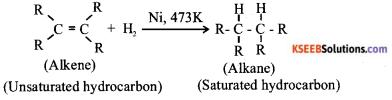
Industrial application:
The process of hydrogenation is used in industry to convert vegetable oils to vanaspathi ghee

The vegetable oil contains a number of unSaturated carbon chain having double bonds between carbon atoms when H2 is bubbled through vegetable oils in presence of nickel as catalyst at 437 K some of the double bonds add H2 to form saturated carbon chain. As a result of this partial hydrogenation, oils are converted into solid fats.
b) Give the names of the following
i) An aldehyde derived from ethane.
ii) Ketone derived from butane
iii) Compound obtained by the oxidation of ethanol by chromic anhydride.
Answer:
i) Ethanal (CH3CHO)
ii) Butanone (CH3CO CH2CH3)
iii) Ethanol(CH3CHO).