Expert Teachers at KSEEBSolutions.com has created Karnataka 1st PUC Chemistry Question Bank with Answers Solutions, Notes, Guide Pdf Free Download of 1st PUC Chemistry Textbook Questions and Answers, Model Question Papers with Answers, Study Material 2020-21 in English Medium and Kannada Medium are part of 1st PUC Question Bank with Answers. Here KSEEBSolutions.com has given the Department of Pre University Education (PUE) Karnataka State Board NCERT Syllabus 1st Year PUC Chemistry Question Bank with Answers Pdf.
Students can also read 1st PUC Chemistry Model Question Papers with Answers hope will definitely help for your board exams.

Karnataka 1st PUC Chemistry Question Bank with Answers
- Chapter 1 Some Basic Concepts of Chemistry
- Chapter 2 Structure of Atom
- Chapter 3 Classification of Elements and Periodicity in Properties
- Chapter 4 Chemical Bonding and Molecular Structure
- Chapter 5 States of Matter
- Chapter 6 Thermodynamics
- Chapter 7 Equilibrium
- Chapter 8 Redox Reactions
- Chapter 9 Hydrogen
- Chapter 10 The s-Block Elements
- Chapter 11 The p-Block Elements
- Chapter 12 Organic Chemistry: Some Basic Principles and Techniques
- Chapter 13 Hydrocarbons
- Chapter 14 Environmental Chemistry
Karnataka 1st PUC Chemistry Syllabus and Marking Scheme
Karnataka 1st PUC Chemistry Blue Print of Model Question Paper
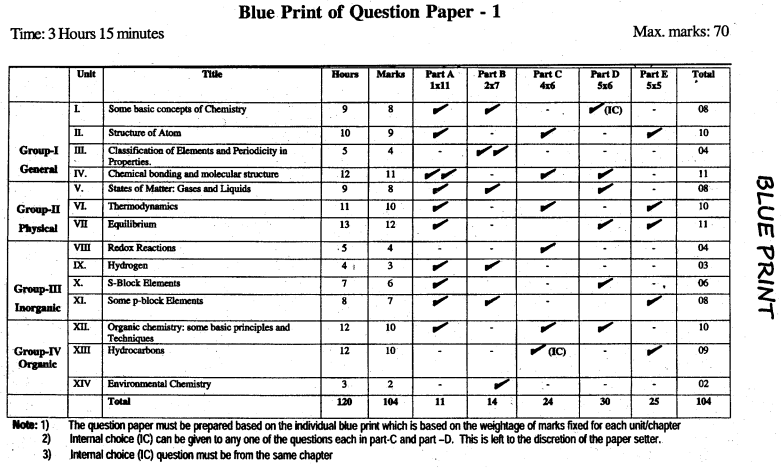
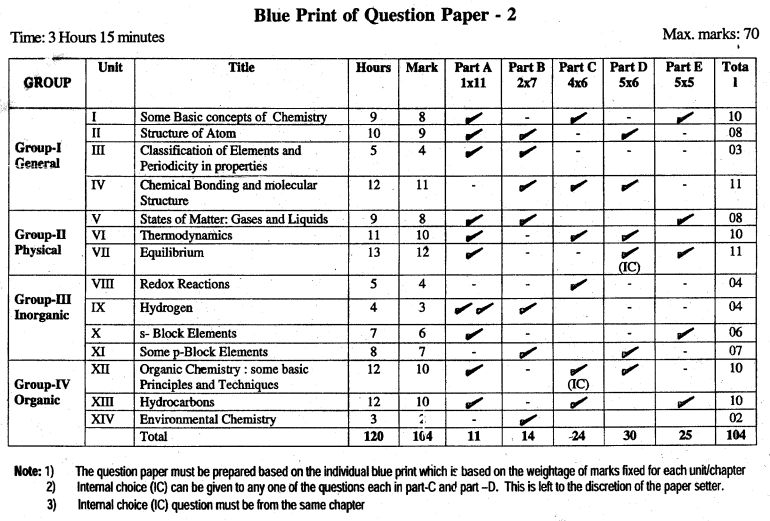
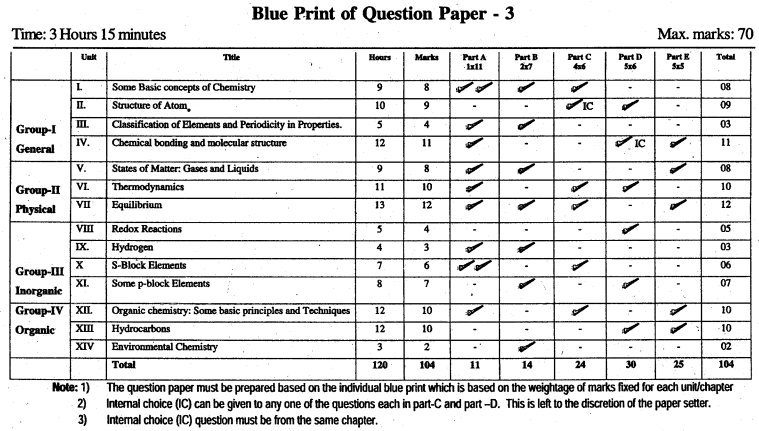
Unit 1 Some Basic Concepts of Chemistry
General introduction, Importance and scope of chemistry, Historical approach to particulate nature of matter, Laws of chemical combination, Dalton’s atomic theory, Atomic and molecular masses, Mole concept and molar mass, Stoichiometry and calculations based on stoichiometry.
Unit 2 Structure of Atom
Discovery of electron, proton and neutron, Atomic number, isotopes and isobars, Rutherford’s model and its limitations, Bohr’s model and its limitations, Concept of shells subshells, Dual nature of matter and light, de Broglie’s relationship, Heisenberg uncertainty principle, Concept of orbitals, Quantum numbers, Shapes of s, p, and d orbitals, Rules for filling electrons in orbitals, Aufbau principle, Pauli’s exclusion principle and Hund’s rule, Electronic configuration of atoms, Stability of half filled and completely filled orbitals.
Unit 3 Classification of Elements and Periodicity in Properties
Significance of classification, Brief history of the development of periodic table; Modern periodic law and the present form of periodic table, Periodic trends in properties of elements; Nomenclature of elements with atomic number greater than 100.
Unit 4 Chemical Bonding and Molecular Structure
Valence electrons, ionic bond and covalent bond, Born Haber Cycle, Polar character of covalent bond, Covalent character of ionic bond, Valance bond theory, VSEPR theory, Concept of hybridization, involving s,p and d orbitals, Shapes of some simple molecules, Molecular orbital theory of homonuclear diatomic molecules, Hydrogen bond.
Unit 5 States of Matter: Gases and Liquids
Three states of matter, Intermolecular interactions, Role of gas laws in elucidating the concept of the molecule, Boyle’s law; Charle’s law, Gay Lussac’s law, Avogadro’s law, ideal behaviour, empirical derivation of gas equation, Avogadro’s number, Ideal gas equation, Liquefaction of gases, Critical temperature, Kinetic energy and molecular speeds, Liquid State – Vapour pressure, viscosity and surface tension.
Unit 6 Chemical Thermodynamics
Concepts of system, types of systems, surroundings, Work, heat, energy, Extensive and intensive properties, State functions, First law of thermodynamics-internal energy change and enthalpy change, Hess’s Law of constant heat summation, Enthalpy of bond dissociation, Enthalpy of combustion, Enthalpy of formation, Enthalpy of atomization, Enthalpy of sublimation, Enthalpy of phase transformation, Enthalpy of ionization and Enthaipy of solution, Introduction of entropy as a state function, Gibbs energy change for spontaneous and nonspontaneous processes, Criteria for equilibrium, Second and third laws of thermodynamics.
Unit 7 Equilibrium
Equilibrium in physical and chemical processes, Dynamic nature of equilibrium. Law of mass action, equilibrium constant, Le Chatelier’s principle, Ionic equilibrium – ionization of acids and bases, Strong and weak electrolytes, Degree of ionization, Ionization of polybasic acids and acid strength, Concept of pH, Henderson Equation, Hydrolysis of salts, Buffer solutions, Solubility product, Common ion effect.
Unit 8 Redox Reactions
Concept of oxidation and reduction, Redox reactions, Oxidation number, Balancing of redox reactions by oxidation number, application of redox reactions.
Unit 9 Hydrogen
Position of hydrogen in periodic table, Occurrence, Isotopes of hydrogen, Preparation properties and uses of dihydrogen, Hydrides-ionic, covalent and interstitial, Physical and chemical properties of water, Preparation and properties of heavy water, Hydrogen peroxide-preparation, properties, structure and uses, Dihydrogen as a fuel.
Unit 10 S-Block Elements (Alkali and Alkaline Earth Metals)
Group 1 And Group 2 Elements:
General introduction, Electronic configuration, Occurrence, Anomalous properties of the first element of each group, Diagonal relationship, Trends in the variation of properties, Trends in chemical reactivity, Preparation and properties of some important compounds.
Unit 11 Some P-Block Elements
General introduction of p-Block Elements, Group 13 elements: General introduction, Electronic configurations, Occurrence, Variation of properties, oxidation states, Trends in chemical reactivity, Anomalous properties of boron, Some important compounds of boron, Aluminium reactions with acids and alkalies and uses. Group 14 elements: General introduction, Electronic configurations, Occurrence, variation of properties, oxidation states, Trends in chemical reactivity, Anomalous behaviour of carbon, Physical and chemical properties: Some important compounds of carbon and silicon.
Unit 12 Organic Chemistry-Some Basic Principles and Techniques
General introduction. Methods of qualitative and quantitative analysis, Classification and IUPAC nomenclature of organic Compounds, Electronic displacement in a covalent bond, Homolytic and heterolytic fission of a covalent bond, Concepts in organic reaction mechanism, Electrophiles and nucleophiles.
Unit 13 Hydrocarbons
General introduction and classification, Alkanes, Alkenes, Alkynes, Aromatic Hydrocarbons, Carcinogenicity and toxicity.
Unit 14 Environmental Chemistry
Environmental pollution, Atmospheric pollution, Greenhouse effect and global warming, Water pollution, Soil pollution, Strategies to control environmental pollution,. Green chemistry.
We hope the given Karnataka 1st PUC Class 11 Chemistry Question Bank with Answers Solutions, Notes, Guide Pdf Free Download of 1st PUC Chemistry Textbook Questions and Answers, Model Question Papers with Answers, Study Material 2020-2021 in English Medium and Kannada Medium will help you.
If you have any queries regarding Karnataka State Board NCERT Syllabus 1st Year PUC Class 11 Chemistry Question Bank with Answers Pdf, drop a comment below and we will get back to you at the earliest.
