Students can Download 2nd PUC Chemistry Model Question Paper 1 with Answers, Karnataka 2nd PUC Chemistry Model Question Papers with Answers helps you to revise the complete Karnataka State Board Syllabus and score more marks in your examinations.
Karnataka 2nd PUC Chemistry Model Question Paper 1 with Answers
Time: 3 Hrs 15 Min
Max. Marks: 70
Part-A
I. Answer all of the following. (Each question carries 1 mark) (10 × 1 =10)
Question 1.
Define VantHaffs factor.
Answer:
VantHaff’s factor is defined as “The ratio of normal molar mass to the abnormal molar mass”.

Question 2.
What are isotonic solutions?
Answer:
Two solutions having same osmotic at a given temperature are isotonic solutions
Question 3.
Mention the S.I unit for molar conductivity.
Answer:
s.m2 mol-1 or s cm2 mol-1
Question 4.
For the reaction A + B → products the rate becomes doubled when concentration of only A is increased by two times, the rate is increased by four times, when the concentration B is alone is doubled what is the order of the reaction?
Answer:
2 or two
![]()
Question 5.
Name the enzyme used in the inversion of cane sugar.
Answer:
Invertase
Question 6.
Name the method used for refining of Zirconium.
Answer:
Van – Arkel method
Question 7.
Complete the reaction
XeF4 + O2 F2 → A + O2 identify A.
Answer:
XeF6
Question 8.
What is a Recemic mixture – ?
Answer:
An equimolar mixture of d and 1 isomrs is called recemic mixture.
Question 9.
Name the product obtained when acetaldehyde reacts with Hydroxyl amine.
Answer:
Acetaldoxime
Question 10.
Name the nitrogenous base present in RNA only.
Answer:
Uracil
Part-B
II. Answer any FIVE of the following. (Each question carries 2 marks) (5 × 2 = 10)
Question 11.
Silver forms CCP lattice and x-ray studies of its crystals show that the edge length of its unit cell is 408.6 pm. calculate the density of silver
Answer:

Question 12.
What is corrosion? Mention a general method to prevent it.
Answer:
The loss of metal in the form of its compounds due to the chemical attack by the environment is called corrosion of metal It is prevented
- By painting
- by alooying by cathodic protection
Question 13.
Write the Arrhenius equation and mention what each term stands for.
Answer:
![]()
K = A.e
Where K = rate constant
A = Arrhenices constant
Ea = Energy of activation
T = Temperature
R = Gas constant
Question 14.
Any two differences between lanthanides and Actinides.
Answer:
Lanthanides
- 4 f – orbital is progressively filled
- Only Pm (promethion) is radio active
- They are less reactive
Actinides
- 5f – orbital os progressively filled
- All are radio active
- They are more reactive
![]()
Question 15.
How does Acetyl chloride react with Anisol in presence of anhydrous aluminum chloride catalyst. Write the chemical equation of the reaction.
Answer:
When acetyl chloride is treated with anisole in the presence of anhydrous AlCl3 catalyst, O and P – acetyl delivatives of anisole is formed.
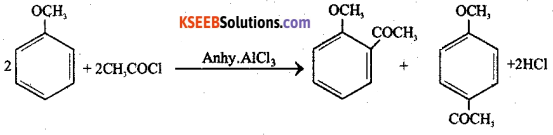
![]()
Question 16.
What are the effects of the electron withdrawing and electron donating groups on acidity of carboxylic acids.
Answer:
Electron donating group decreases acidic strength and electron with drawing group increases acidic strength of carboxylic acids.
Question 17.
What are antacids? Give an example.
Answer:
The chemical substances which are used in the treatment of acidity are called antacids.
Ex: Ranitidine (Zantac)
Question 18.
What are food preservatives? Give one example.
Answer:
The chemical substances which prevent spoilage of food due to microbial growth are called food preservatives.
Ex: Table salt, sugar, Vegetable oils.
Part – C
III. Answer Any FIVE of the following. (Each question carries three marks) (5 × 3 = 15)
Question 19.
How is pure alumina obtained from Bauxite by leaching process?
Answer:
1. Bauxite contains impurities like
TiO2 – Fe2O3 etc.
Bauxite is concentrated by digesting the powdered ore in a concentrated solution of NaOH at 473 – 573 K and 35 bar pressure. Al2O3 is leached as sodium aluminate.
The insoluble Fe2O3 and TiO2 are removed by filtration.
H2O3 + α NaOH + 3H2O → 2Na [A1 (OH)4]
2. The solution of sodium aluminate is nutralized by passing CO2 gas and the solution is seeded with treshly prepared Al2O3.
2Na[Al(OH)4] + CO2 → Al2O3 x H2O + 2NaHCO3
3. Hydrated alumina is filtered dried and heated to get pure Alumina Al2O3
![]()
Question 20.
Write the reactions that takes place during the manufacture of nitric acid by Ostwald’s process.
Answer:
Manufacture of HNO3 by ostwald’s process involves 3 steps.
Step – 1: Oxidation of ammonia to NO
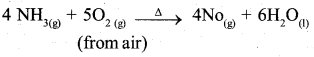
Step – 2 : Oxidation of No to NO2
2NO(g) + O2(g) → 2NO2(g)
Step – 3,
3NO2(g) + H2O(I) → 2 HNO3(q) + NO(g)
![]()
Question 21.
1. What happens when potassium chlorate is heated in presence of MnO2. Write the equation for the reaction also.
Answer:
When potassium chlorate is heated in the presence of MnO2 as a catalyst at 420K, oxygen is liberated.
![]()
2. draw the structure of sulphuric acid.
The structure of H2SO4 is

Question 22.
1. How is chlorine prepared by using MnO2?
Answer:
Chlorine is prepared by heating MnO2 with con HCl.
MnO2 +4HCl → MnCl2 + Cl2 + 2H2O
2. Complete the reaction NH3 + Cl2 (excess) →
NH3 + Cl2 (excess) → NCl3 + 3HCl
Question 23.
d-block elements form co-ordination compounds. Give reasons.
Answer:
d-block elements form co-ordination compounds.
Reasons :
- They are small size of metals ions
- They have high ionic charges,
- Availability of d – orbitals
Question 24.
How is potassium dichromate pre¬pared from the chromite ore?
Answer:
The manufacture of K2Cr2O7 from chromite ore involves is steps.
Step -1: 4 FeCr2 O4 + 8Na2CO3 + 7O2 → 8Na2 CrO4 + 2FerO3 + 8CO2
Step – 2 : 2Na2CrO4 + 2H+ → Na2Cr2O7 + 2Na+ + H2O
Step – 3 : Na2Cr2O7 + 2Kcl → K2Cr2O7 + 2NaCl
OR
Complete explanation in 3 steps
Question 25.
Mention the geometry, magnetic property and type of hybridization in [Ni(cn)4]2- complex.
Answer:
Geometry : square planar complex Magnetic property: Diamagnetic Hybridisation: ASP2
Question 26.
Write any three postulates of Werner’s theory of complexes.
Answer:
- Co-ordination compounds metals show two types of Valencies primary and secondary
- Primary valencies are ionisable
- Secondary valencies are non-ionisable
![]()
Part-D
IV. Answer any THREE of the following. [Each question carries 5 marks. (3 × 5 = 15)
Question 27.
a) Calculate the packing efficiency in body centered cubic crystals. (3)
Answer:
In a BCC unit cell the edge length is ‘a’ and radius of the particle is ‘r’. Then r = \(\sqrt{3} / 4 \mathrm{a}\) and two particles are present in an unit cell of BCC.
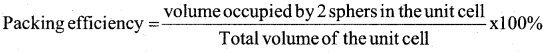
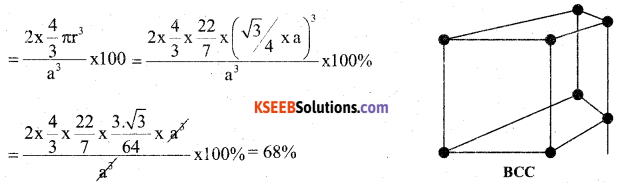
b. Calculate the number of particles per unit cells in f.c.c. (2)
No of particles per unit cell in
fcc = \(\frac { 1 }{ 8 }\) × 8 + \(\frac { 1 }{ 2 }\) × 6 =1 + 3=4
Question 28.
a) The boiling point of benzene is 353.23K. When 1.80 g of a non volatile, non electrolytic solute was dissolved in 90 g of benzene, the boiling point was raised to 354.11 K calculate the molar mass of the solute
[Kb for benzene = 2.53 K.kg mol-1] (3)
Answer:
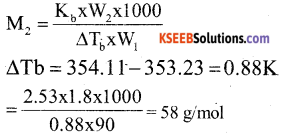
(b) Write two differences between ideal and non ideal solutions of two liquids (2)
Question 29.
a) Calculate the standard free energy change for the following reaction occurring in the galvanic cell at 298K
3Mg(S) + 2Al3+ (aq) → 3Mg2+ (aq) + 2 Al(S)
Given E0Mg2+/Mg = -2.37 V and E0Al3+/Al = -1.66V (4)
Answer:
Ecell = E0R – E0L
= -1.66 + 2.37
= 0.71 V
ΔG0 = -nFE0cell
= -6 × 96500 × 0.71
= -411090 J or -411.09 KJ
b) What is primary battery?
Answer:
A battery which cannot be recharged and revised is called a primary battery.
![]()
Question 30.
a) Derive the integrated rate equation for the rate constant of a first order reaction. (4)
Answer:
Consider a first order reaction R → P

On integration, we get d[R]
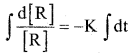
In [R] = -Kt + I …………. (1)
Where I = Integration constant To calculate I,
t = 0,[R] = [R]0
In [R]0 = -k x 0 + I
I = in [R]0
Substitute the value of I in eq2 …………. (1)
In [R] = -Kt + In [R]0
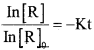

b) What is pseudo first order reaction? (1)
Answer:
A Chemical reaction in which order is one but molecularity is two or more is called pseudo unimolecular reaction.
Question 31.
a) Complete and balance the following reactions
SO2 + H2S oxidation →
![]()
FeCl3 + H2O hydrolysis → (2)
![]()
b) Mention two characteristics of enzyme catalysis (2)
Answer:
- Highly efficient
- Highly specific in nature
c) What is the sign of AS for the adsorption of gas on solids?
Answer:
ΔS decreases or ΔS = -Ve
Part – D
V. Answer any FOUR of the following. (Each question carries 5 marks) (4 × 5 = 20)
Question 32.
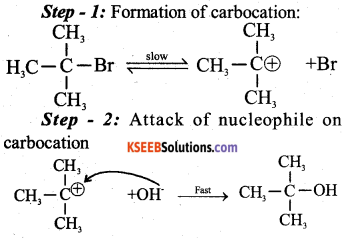
a) Explain SN1 mechanism by taking tertiary butyl bromide as an example.(3)
Answer:
SN1 mechanism involves two steps
b) What is Wurtz Fitting’s reaction? Give an example. (2)
Answer:
Alkyl halide reacts with aylhalide in the presence of sodium in dry ether gives alkyl arenes.

![]()
Question 33.
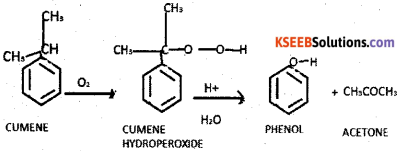
a) How is phenol manufactured by cumene process. Give the chemical equations of the reaction involved. (3)
Answer:
Cumene is oxidised by oxygen to form cumene hydroperoxide. Which on acid hydrolysis gives phenol.
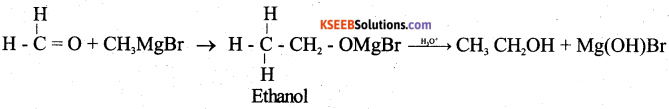
b) How do you prepare ethanol by using the Grignard Reagent? (2)
Answer:
When Grignard reagent is treated with HCHO addition product is formed which on hydrolysis gives ethanol
Question 34.
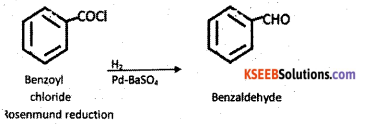
a) How is Benzol chloride converted into benzaldehyde? Name the reaction. (3)
Answer:
When benzoyl chloride on reduction with H2 in the purence of palladium catalyst supported on barium sulphate benzaldehyde is formed
b) Write the chemical equation for the reaction between dilute NaOH and acetalhyde, mention the name of the product formed. (2)
Answer:
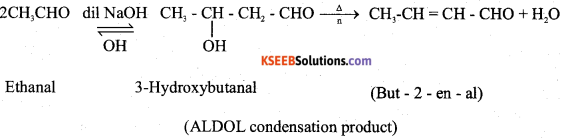
![]()
Question 35.
a) Explain how is Hinberg’s reagent is used to distinguish the primary, secondary and tertiary amines.
Answer:
Hinsberg’s reagent reacts with 10 amine gives a product which is soluble in alkali.
Hinsberg’s reagent reacts with 20 amine gives a product which is insoluble in alktali.
Hinsberg’s reagent doesnot react with 30 amine.
b) Write the chemical reactions involved in the conversion of aniline into phenol (2)
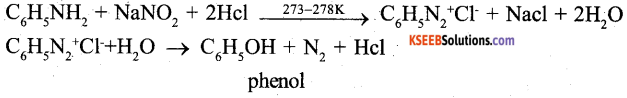
Question 36.
a) What are carbohydrates? and how are they classified? (3)
Answer:
Carbohydrates are optically active polyhydroxy aldehydes or polyhydroxy ketones or those compounds which yield these on hydrolysis carbohydrates are classified into 3 types namely, monosaccharides, oligosaccharides and polysaccharides,
b) What is a peptide bond? How many peptide bonds are present in a tetra peptide?(2)
Answer:
The -CONH – bond formed b/w two α – amino acids is called peptide bond.
![]()
3 peptide bonds
Question 37.
a) 1. What are condensation polymers? Give an example.
Answer:
Condensation reacts b/w two different bifunctional or trifunctional monomeric units to give a polymer with the loss of simple molecules like H2O NH3 etc. is called condensation polymerisation. The polymers obtained are called condensation polymers.
Ex: Nylon 6.6, Dacron etc.
2. Give the IUPAC name of the monomer of natural rubber. (3)
Answer:
Isoprene or 2 – methyl – 1,3 – butadiene,
b) What are Biodegradable polymers? Give an example. (2)
Answer:
The polymers which decompose on it own during a certain period of time are called biodegradable polymers.
Ex : PHBV, Nylon – 2 – hycon – 6.